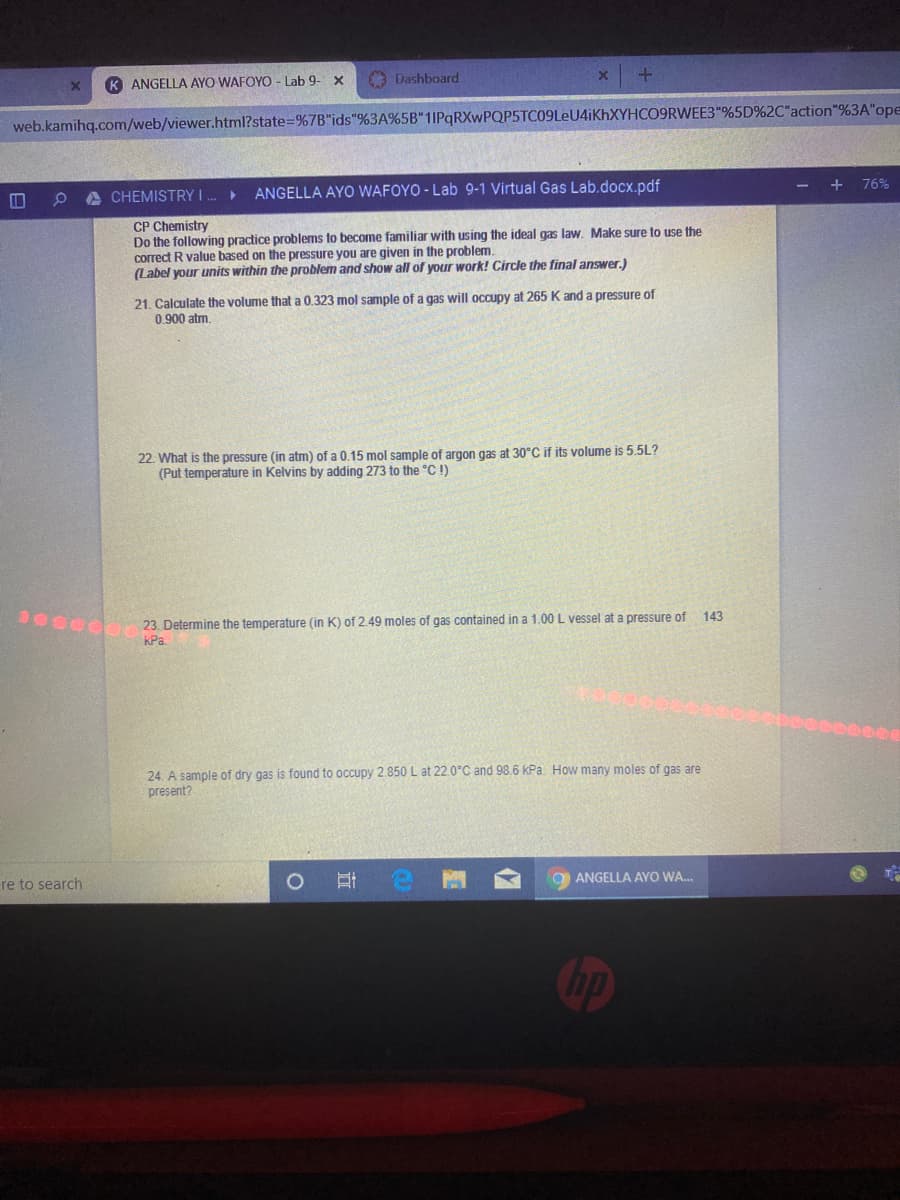
+ 76% CHEMISTRY I . ► ANGELLA AYO WAFOYO - Lab 9-1 Virtual Gas Lab.docx.pdf CP Chemistry Do the following practice problems to become familiar with using the ideal gas law. Make sure to use the correct R value based on the pressure you are given in the problem. (Label your units within the problem and show all of your work! Circle the final answer.) 21. Calculate the volume that a 0.323 mol sample of a gas will occupy at 265 K and a pressure of 0.900 atm. 22. What is the pressure (in atm) of a 0.15 mol sample of argon gas at 30°C if its volume is 5.5L? (Put temperature in Kelvins by adding 273 to the "C !) 143 23. Determine the temperature (in K) of 2.49 moles of gas contained in a 1.00 L vessel at a pressure of KPa.
+ 76% CHEMISTRY I . ► ANGELLA AYO WAFOYO - Lab 9-1 Virtual Gas Lab.docx.pdf CP Chemistry Do the following practice problems to become familiar with using the ideal gas law. Make sure to use the correct R value based on the pressure you are given in the problem. (Label your units within the problem and show all of your work! Circle the final answer.) 21. Calculate the volume that a 0.323 mol sample of a gas will occupy at 265 K and a pressure of 0.900 atm. 22. What is the pressure (in atm) of a 0.15 mol sample of argon gas at 30°C if its volume is 5.5L? (Put temperature in Kelvins by adding 273 to the "C !) 143 23. Determine the temperature (in K) of 2.49 moles of gas contained in a 1.00 L vessel at a pressure of KPa.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 102GQ: Cloth can be waterproofed by coating it with a silicone layer. This is done by exposing the cloth to...
Related questions
Question
100%
Transcribed Image Text:K ANGELLA AYO WAFOYO- Lab 9- x
Dashboard
web.kamihq.com/web/viewer.html?state=%7B"ids"%3A%5B"1IPqRXwPQP5TC09LeU4iKhXYHCO9RWEE3"%5D%2C"action"%3A"ope
+
76%
A CHEMISTRY I.
ANGELLA AYO WAFOYO - Lab 9-1 Virtual Gas Lab.docx.pdf
CP Chemistry
Do the following practice problems to become familiar with using the ideal gas law. Make sure to use the
correct R value based on the pressure you are given in the problem.
(Label your units within the problem and show all of your work! Circle the final answer.)
21. Calculate the volume that a 0.323 mol sample of a gas will occupy at 265 K and a pressure of
0.900 atm.
22. What is the pressure (in atm) of a 0.15 mol sample of argon gas at 30°C if its volume is 5.5L?
(Put temperature in Kelvins by adding 273 to the °C !)
143
S 23 Determine the temperature (in K) of 2.49 moles of gas contained in a 1.00 L vessel at a pressure of
KPa
24. A sample of dry gas is found to occupy 2.850 L at 22.0°C and 98.6 kPa. How many moles of gas are
present?
ANGELLA AYO WA.
re to search
bp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning