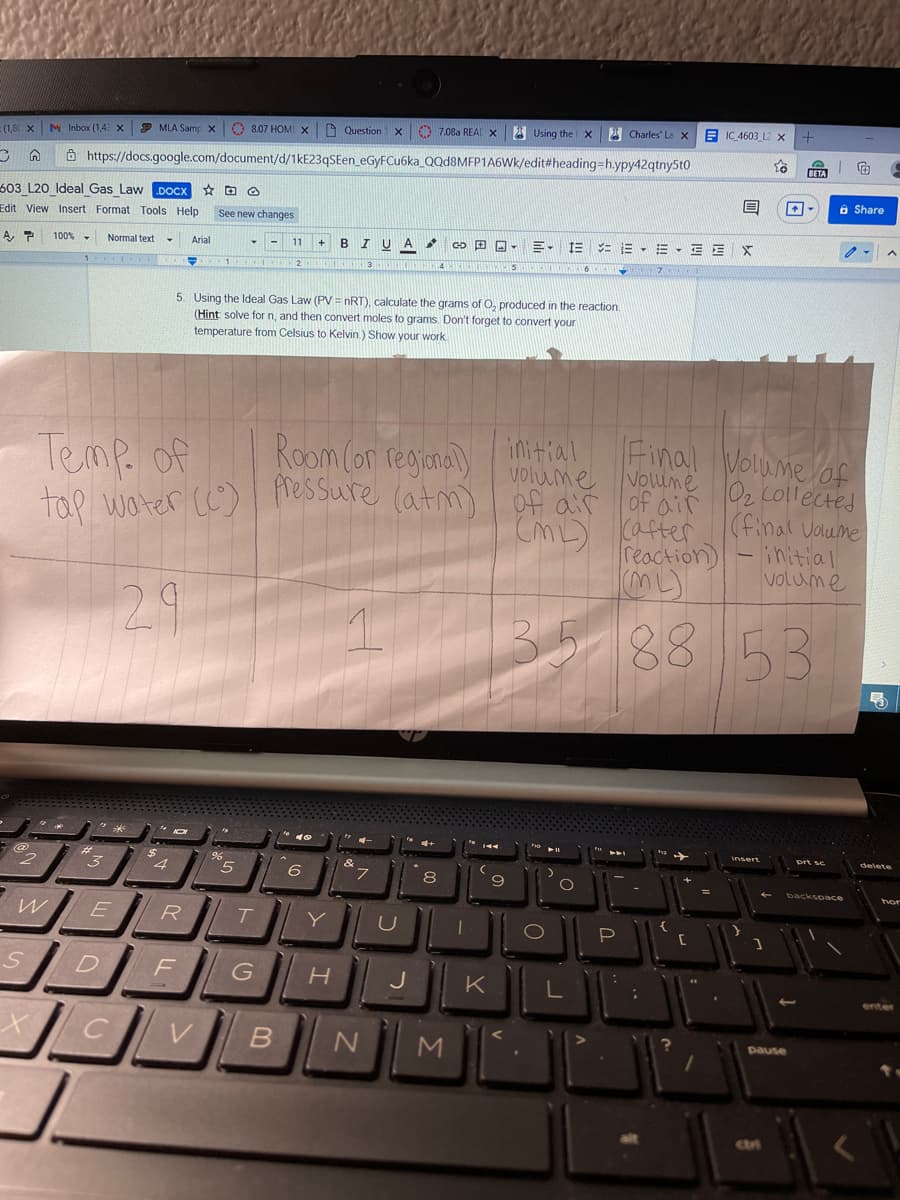
BIUA E, E = E - E - E E X 100% - Normal text Arial 11 5. Using the Ideal Gas Law (PV = nRT), calculate the grams of O, produced in the reaction. (Hint solve for n, and then convert moles to grams. Don't forget to convert your temperature from Celsius to Kelvin.) Show your work Final Volume lof Voume 02, collected Room (or regiana) initial Temp. of tap water (C) Volume fressure (atm) of air of air cafter reaction) (final vaume initial volume 2.9 35-188
BIUA E, E = E - E - E E X 100% - Normal text Arial 11 5. Using the Ideal Gas Law (PV = nRT), calculate the grams of O, produced in the reaction. (Hint solve for n, and then convert moles to grams. Don't forget to convert your temperature from Celsius to Kelvin.) Show your work Final Volume lof Voume 02, collected Room (or regiana) initial Temp. of tap water (C) Volume fressure (atm) of air of air cafter reaction) (final vaume initial volume 2.9 35-188
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 8E: Predict and test the behavior of a particles fired at a plum pudding model atom. Predict the paths...
Related questions
Question
Answer question above and show all work
Transcribed Image Text:O 8.07 HOME x Question
O 7.08a REAL x A Using the X
A Charles' La xE IC 4603 L2 x
E(1,80 x
M Inbox (1,43
P MLA Samp X
ô https://docs.google.com/document/d/1kE23qSEen_eGyFCuáka_QQ48MFP1A6WK/edit#heading-h.ypy42qtny5t0
BETA
603 L20 Ideal Gas Law
A Share
DOCX
Edit View Insert Format Tools Help
See new changes
A, ?
B I
U A
IE = E E - E E
100% -
Normal text
Arial
11
5. Using the ldeal Gas Law (PV = nRT), calculate the grams of O, produced in the reaction.
(Hint solve for n, and then convert moles to grams. Don't forget to convert your
temperature from Celsius to Kelvin.) Show your work
Final Volume of
Voume
of air
cafter
reaction)
Temp. of
Room (or reginal)
initial
Volume
O2 Collected
(final vaume
initial
Volume
top woter (c) Hessure (atm) of air
29
3588
53
insert
delete
4
6
9
R
Y
FG
J
K
enter
pause
cir
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning