8) Compare the values obtained for the pressure(atm) of 2 mols Oz at 298.15K held in a 8.25 dm bulb using a) Redlick-Kwong equation: For O2, Tc = 154.6 K and Pc = 50.43 bar b) Beattie – Bridgeman equation
8) Compare the values obtained for the pressure(atm) of 2 mols Oz at 298.15K held in a 8.25 dm bulb using a) Redlick-Kwong equation: For O2, Tc = 154.6 K and Pc = 50.43 bar b) Beattie – Bridgeman equation
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.39E
Related questions
Question
Show complete solutions and enclose all final answers in a box. Round off final answers to 4 decimal places and floating values for intermediate answers. Use 2 decimal places for molecular weights.
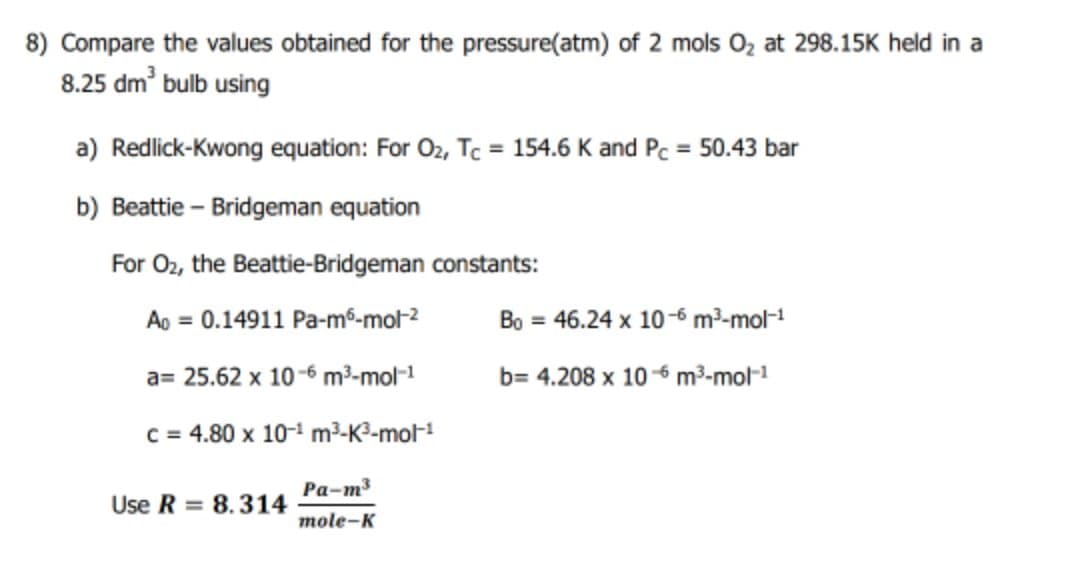
Transcribed Image Text:8) Compare the values obtained for the pressure(atm) of 2 mols 0z at 298.15K held in a
8.25 dm' bulb using
a) Redlick-Kwong equation: For 02, Tc = 154.6 K and Pc = 50.43 bar
%3D
b) Beattie – Bridgeman equation
For O2, the Beattie-Bridgeman constants:
Ao = 0.14911 Pa-m6-mol2
Bo = 46.24 x 10-6 m²-mol-
a= 25.62 x 10 -6 m³-mol-1
b= 4.208 x 106 m³-mol-1
C = 4.80 x 10-1 m3-K3-mol1
Ра-т3
Use R = 8.314
mole-K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,