1. The Maxwell-Boltzmann distribution of speeds is 3 mv² v²e¯2kT т f (v) = 47 a) Calculate the fraction of CO2 molecules at 300K between 100 and 105 m/s. b) Repeat for 600 and 605 m/s. c) Repeat a) and b) at T=1000K.
Q: P1C.12 The critical constants of a van der Waals gas can be found by setting the following…
A: GIVEN: To Calculate: Vc=? Pc=? Tc=?
Q: P1C.13 A scientist proposed the following equation of state: RT В C Vm V? V3 m m Show that the…
A:
Q: (a) Find the average rate of change of P as V increases from 200 in3 to 250 in3. (b) Express V as a…
A: (a)
Q: calculate the RMS velocity in m/s for an imaginary gas with a molar mass of 46.96 g/mol at 228.9 K…
A: Given that, Molar mass = 46.96 g mol-1 Temperature = 228.9 K
Q: 13.1 liter steel tank contains 0.4 kg of Argon gas. If the maximum temperature that this tank can…
A:
Q: |char E1A.11(a) A vessel of volume 22.4 dm’ contains 2.0 mol H,(g) and 1.0 mol P/ ´N,(g) at 273.15K.…
A: Given data Moles of H2 = 2 Moles of N2 = 1 Temperature = 273.15 K Volume = 22.4 dm3 = 22.4 L Gas…
Q: 4B. The value of the compression factor (Z) approaches 1 for all real gases in the limit as (explain…
A: Compression factor is defined as the factor which corrects the deviation of any real gas from the…
Q: 1. Determination of the Ideal Gas Constant "R" using Carbon Dioxide 10 °C- 3700ml 768.0mm Hg…
A: As per student request Part 1(First question) Solved!!
Q: SO2 can be defined by the Van der Waals equation of state with constants a = 6.86 bar*L?/mol? and b…
A: The critical temperature, Tc, pressure, Pc, and volume, Vc is calculated as shown below.…
Q: The density of air under normal conditions (temperature To-273 K, pressure po- 100 kPa) is 1.29 kg/…
A: The number of moles is a ratio of the mass of a substance to the molar mass of the substance.…
Q: What is the average velocity (m/s) of a molecule of hydrogen at 200.4K? Do not type units into your…
A: Given Temperature = 200.4°K
Q: Ammonia gas (A) diffuses through a stagnant gas mixture consisting of one-third Nitrogen (B) and two…
A:
Q: At a temperature of 300K and a certain pressure, the mean free path (l) of oxygen molecules is 0.1…
A: Collisional frequency is the average rate of collisions between two reactants for a particular…
Q: Use the kinetic model to calculate the root-meansquarespeed of (a) N2 (b) H20 molecules in the…
A: Root mean square speed: Root mean square speed is given by formula, μ(rms)=3RTM Where, R=gas…
Q: The collision diameter of N2 is 3.74 x 10-10 m at 298.15 K and 101.325 kPa. Its average speed is…
A:
Q: Calculate the most probable speed, vmp ( in m s-1) of He molecules at 25.0oC, 1.00 bar. Answer in…
A:
Q: Given the following gas Component Volume (%) Methane 58.7 Ethane 16.5 Propane 9.9 Butanes 5.0…
A: Kindly get the answer given below.
Q: A certain gas obeys the van der Waals equation with a= 0.50 m6 Pa mol-2. Its volume is found to be…
A: The equation which gives the relationship between volume, pressure, the amount, and temperature of…
Q: Boyle's Law states that when a sample of gas is compressed at a constant temperature, the pressure P…
A: According to Boyle's law, at constant temperature and moles of gas, Pressure of gas varies inversely…
Q: 1. Determination of the Ideal Gas Constant "R" using Carbón Dioxidê 10 °C. Volume of plastic bag…
A: Ideal gas equation, PV =nRT R, gas constant = PVnT where P= pressure V= volume n = moles of gas T…
Q: Q2. The compression factor of ethene (g) is 0.80 at 400 K and 260 bar. What is the value of…
A:
Q: Boyle's Law states that when a sample of gas is compressed at a constant temperature, the pressure P…
A: Thus volume is going to be decreasing at the rate of -100cm3min-1 .
Q: (ii) Calculate the fraction of oxygen molecules moving with a speed between 700 ms" and 710 ms' at…
A: Hello. Since your question has multiple parts, we will solve the first question for you. If you want…
Q: The best laboratory vacuum pump can generate a vacuum of about 1 nTorr. At 25oC and assuming air…
A: P = 1 ntorr = 1 x 10-9 torr = 1.33 x 10-7 Pa T = 25oC = 298 K d = 395 pm = 395 x 10-12 m a).…
Q: Given the van der Waals constants for ethane gas as a = 5.562L2bar/mol2, b = 0.06380L/mol, for 10.0…
A:
Q: -42 cm/mol for CH and B = -732 8.6 At 25°C, B cm/mol for n-C,H0. For a mixture of 0.0300 mol of CH,…
A: given data are mixture of 0.0300 mol of CH4 and 0.0700 mol of n-C4H10 so n= no. of moles =…
Q: 2) a) Virial equation of state of real gases is expressed as Z = (PV / nRT) = 1 + (nB / V) + (n2C /…
A: Vander Waals equation for a real gas can be written as follow: P+aVm2=RTVm-b where P is pressure, Vm…
Q: 7. A sample of 1.00 mole of Xe gas occupies 25.00dm at 200.00K. If treated as a perfect gas, the…
A: For an ideal gas z = 1 (as no intermolecular attractive or repulsive interactions exist). For a real…
Q: At an altitude of 15 km the temperature is 217 K and the pressure 12.1 kPa. What is the mean free…
A:
Q: An unknown gas (with PVT conditions of; 101 kPa, 2.83 m³ & 23°C) is to be transferred into a storage…
A: Given data,
Q: Virial Equation of State The virial equation of state: Z = PVm RT - 1 + B(T) C(T) Vm + V2 m + D(T)…
A:
Q: For a mixture of 0.0262 mol of C2H4 and 0.1183 mol of CO2 in a 621.7 cm³ container at 574.67 K,…
A: Given: 0.0262 mol of C2H4 0.1183 mol of CO2 Volume = 621.7 cm3 Temperature = 574.67 K Z = 0.9268
Q: Calculate the fraction of N2 molecules with speed at 550K in the range of 300 m/s to 320 m/s.
A:
Q: Assume that the average molecular velocity v of a gas in a particu- lar container is given by v(T) =…
A: Given: v(T) = 29T And T = 200 P Where T = Temperature And P = pressure
Q: A sample of 0.200 mol Cl2(g) is held in a container of volume 250 cm3 At 500 K, the gas exerts a…
A: Given: The number of moles of Cl2 (g) is 0.200 mol. The volume of the container is 250 cm3. At 500 K…
Q: for molecules of 02 at a temperature at OCelcius and a preasure of 1 atm (101325 Pa). Calculate the…
A: Number of collisions per second means collision frequency. Collision frequency(v) is calculated…
Q: For a mixture of 0.0549 mol of C2H4 and 0.1277 mol of CO2 in a 694.5 cm3 container at 668.8 K,…
A:
Q: Calculate the pressure exerted by 1.0 mol C2H6 behaving as (a) a perfect gas, (b) a van der Waals…
A: The perfect gas is an ideal gas that obeys the ideal gas law. It defines as the one in which the…
Q: What is the root-mean-square speed (in m/s) of methyl mercaptan gas (CH3SH) at 68°F? Show work for…
A: Root mean square speed of a gas is given by the expression RMS=3RTM where R is the ideal gas…
Q: Boyle's Law states that when a sample of gas is compressed at a constant temperature, the pressure P…
A: Bolye’s law states that the pressure of an ideal gas is inversely proportional to its volume.The…
Q: Only answer letters (d) and (e) Nitrogen is maintained at 152 kPa in a 2.00-dm3 vessel at 298.15K.…
A: d) Calculation of average translational energy: Ek=12murms2=12×28.0134×10-3 kg mol-16.022×1023…
Q: Estimate the number of collisions per second for an oxygen molecule (M=32.0 g/mol) with speed%3Dvms…
A:
Q: The collision diameter of N2 is 3.74 x 10-10 m at 298.15 K and 101.325 kPa. Its average speed is…
A: Given data: Collision diameter of N2 = 3.74 x 10-10 m Temperature = 298.15 K…
Q: The molecular weight of two components A and B of a gas mixture are 24 and 28 respectively. The…
A: According to the question we have two components A and B. The information about the two components…
Q: Methane molecules, CH4, may be considered as spherical, with a collision cross-section of σ = 0.46…
A: Given: The collision cross-section σ=0.46 nm2 The given molecule is methane. To find: The value of…
Q: The Dieterici equation of state is similar to the van der Waals equation in that they both employ…
A:
Q: re consists of 320 mg of methane, 175 mg of argon, and 225 mg of neon. e partial pressure of neon at…
A: Ideal gas assumption is:
Q: For a diatomic carbon dioxide gas (CO2 molar mass is 44.0g/mol) at T=300K, calculate (a) the most…
A: Velocity order will be Vrms> Vav > Vmp
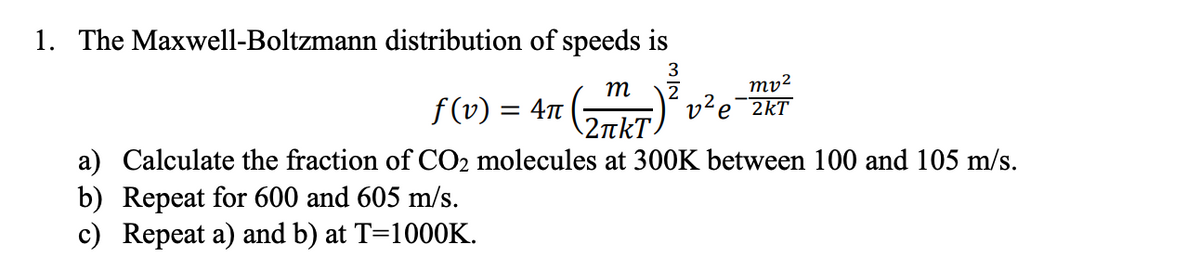
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- A specially constructed velocity-selector accepts a beam of molecules from an oven at a temperature T but blocks the passage of molecules with a speed greater than the mean. What is the mean speed of the emerging beam, relative to the initial value? Treat the system as one-dimensional.The best laboratory vacuum pump can generate a vacuum of about 1 nTorr. At 25 °C and assuming that air consists of N2 molecules with a collision diameter of 395 pm, calculate at this pressure (i) the mean speed of the molecules, (ii) the mean free path, (iii) the collision frequency in the gas.Given the van der Waals constants for ethane gas as a = 5.562 L2bar/mol2, b = 0.06380L/mol, for 10.0 mol of ethane at 300 K and under 30 bar, [9]i. Calculate the second virial coefficient B at this temperature.ii. What does this imply about the nature of the interactions between themolecules at this temperature?iii. Calculate the compression factor (Z) from the first two terms of the virialequation of state.iv. Estimate the molar volume from Z
- The molar volume of oxygen, O2, is 3.90 dm3 mol-1 at 10.0 bar and 200 °c. Assuming that the expansion may be truncated after the second term, calculate the second virial coefficient of oxygen at this temperature.P1A.5 Deduce the relation between the pressure and mass density, ρ, of a perfect gas of molar mass M. Confirm graphically, using the following data on methoxymethane (dimethyl ether) at 25 °C, that perfect behaviour is reached at low pressures and the molar mass of the gas. p/kPa 12.223 25.20 36.97 60.37 85.23 101.3 ρ/(kgm–3) 0.225 0.456 0.664 1.062. 1.468 1.734Calculate the fraction of N2 molecules with speed at 550K in the range of 300 m/s to 320 m/s.
- Estimate the percentage difference between the van der Waals and perfect gas calculations for carbon dioxide undergoing a change from 2.0 bar to 60 bar at 25° C. carbon dioxide: a=3.610 atmdmmol, b=0.0429 dm mol, 1.0atm=1.013×10 Pa, R=8.3145JK mol-1 ( Δ Gvdw- Δ Gideal)/ Δ Gideal = ______%For a diatomic carbon dioxide gas (CO2 molar mass is 44.0g/mol) at T=300K, calculate (a) the most probable speed (b) the average speed and (c) the root-mean-square speedNe(g)Ne(g) effuses at a rate that is ______ times that of Xe(g)Xe(g) under the same conditions.
- For an ideal gas obeying the ideal gas law, P = nRT/V , where R is the gas constant. Write the total differential dz and evaluate the partial derivativesIn general, the compressibility factor Z tends to be larger than 1 at very high pressures (greater than 350 bar for methane.) Which Van der Waals' factor seems to be responsible for this behavior.--- a or b? ExplainAt 300K, the virial coefficients (B) of N2 and CH4 are -4.2cm^3 mol^-1 and -15cm^3 mol ^-1, respectively. Which gas behaves more ideally at this temperature?

