8.6 At 25°C, B = = -732 cm/mol for -C,H10: For a mixture of 0.0300 mol of CH, and 0.0700 mol of n-C,H at 25°C in a 1.000-L vessel, calculate the pressure using the virial equation and (a) the approximation B12 (B, + B,); (b) the fact that for this mixture, B1, = – 180 cm/mol. Compare the results with the ideal-gas-equation -42 cm/mol for CH, and B result.
8.6 At 25°C, B = = -732 cm/mol for -C,H10: For a mixture of 0.0300 mol of CH, and 0.0700 mol of n-C,H at 25°C in a 1.000-L vessel, calculate the pressure using the virial equation and (a) the approximation B12 (B, + B,); (b) the fact that for this mixture, B1, = – 180 cm/mol. Compare the results with the ideal-gas-equation -42 cm/mol for CH, and B result.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 45QRT: At the critical point for carbon dioxide, the substance is very far from being an ideal gas. Prove...
Related questions
Question
Plz solve it in detail and with explanation
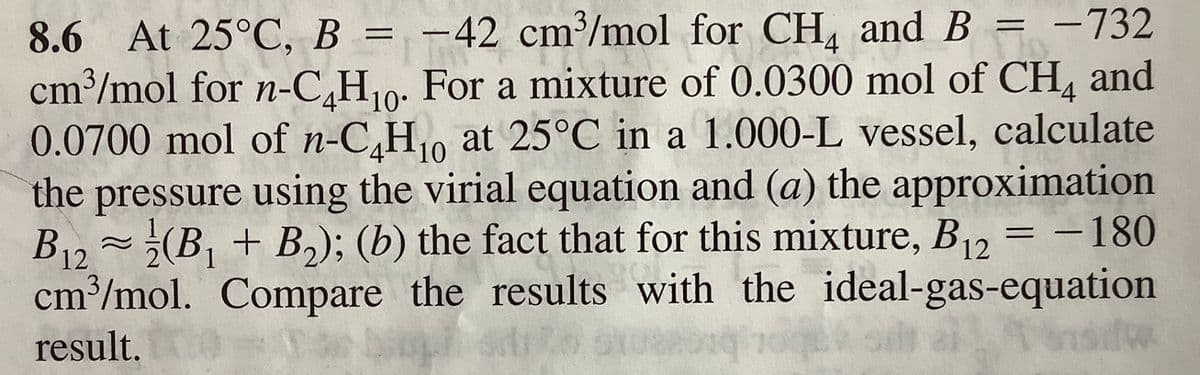
Transcribed Image Text:8.6 At 25°C, B = -42 cm³/mol for CH, and B = -732
cm³/mol for n-C,H10: For a mixture of 0.0300 mol of CH, and
0.0700 mol of n-C,H0 at 25°C in a 1.000-L vessel, calculate
the pressure using the virial equation and (a) the approximation
B12~¿(B, + B,); (b) the fact that for this mixture, B = -180
cm³/mol. Compare the results with the ideal-gas-equation
Ponsidw.
%3D
4
12
result.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 99 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,