9. By employing our understanding of nucleophiles and electrophiles, as well as kinetics and thermodynamics, we should be able to predict the electron-pushing arrow mechanisms, and the corresponding products, for reactions that we have not explicitly discussed in class. An example of this is the two-step addition of a hydrohalic acid (HX) across a pi bond, wherein the pi bond behaves as a the electron donor. However, as shown in the example below, there often exists the possibility of obtaining more than one product: H-Br Br A Br Br a. Using the pi bond between carbons 1 & 2 as the donor electrons, draw complete electron-pushing arrow mechanisms that account for the formation of each product (a-c) above. It is advisable for you to count your atoms in both the stating material and products. b. The formation of which product(s) above is kinetically favored? How do you know? c. The formation of which product(s) above is thermodynamically favored? How do you know? d. Sketch a Gibbs Free Energy Diagram that reflects your answers from parts (b) & (c).
9. By employing our understanding of nucleophiles and electrophiles, as well as kinetics and thermodynamics, we should be able to predict the electron-pushing arrow mechanisms, and the corresponding products, for reactions that we have not explicitly discussed in class. An example of this is the two-step addition of a hydrohalic acid (HX) across a pi bond, wherein the pi bond behaves as a the electron donor. However, as shown in the example below, there often exists the possibility of obtaining more than one product: H-Br Br A Br Br a. Using the pi bond between carbons 1 & 2 as the donor electrons, draw complete electron-pushing arrow mechanisms that account for the formation of each product (a-c) above. It is advisable for you to count your atoms in both the stating material and products. b. The formation of which product(s) above is kinetically favored? How do you know? c. The formation of which product(s) above is thermodynamically favored? How do you know? d. Sketch a Gibbs Free Energy Diagram that reflects your answers from parts (b) & (c).
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter8: Haloalkanes, Halogenation, And Radical Reactions
Section: Chapter Questions
Problem 8.34P
Related questions
Question
100%
Please answer all parts:
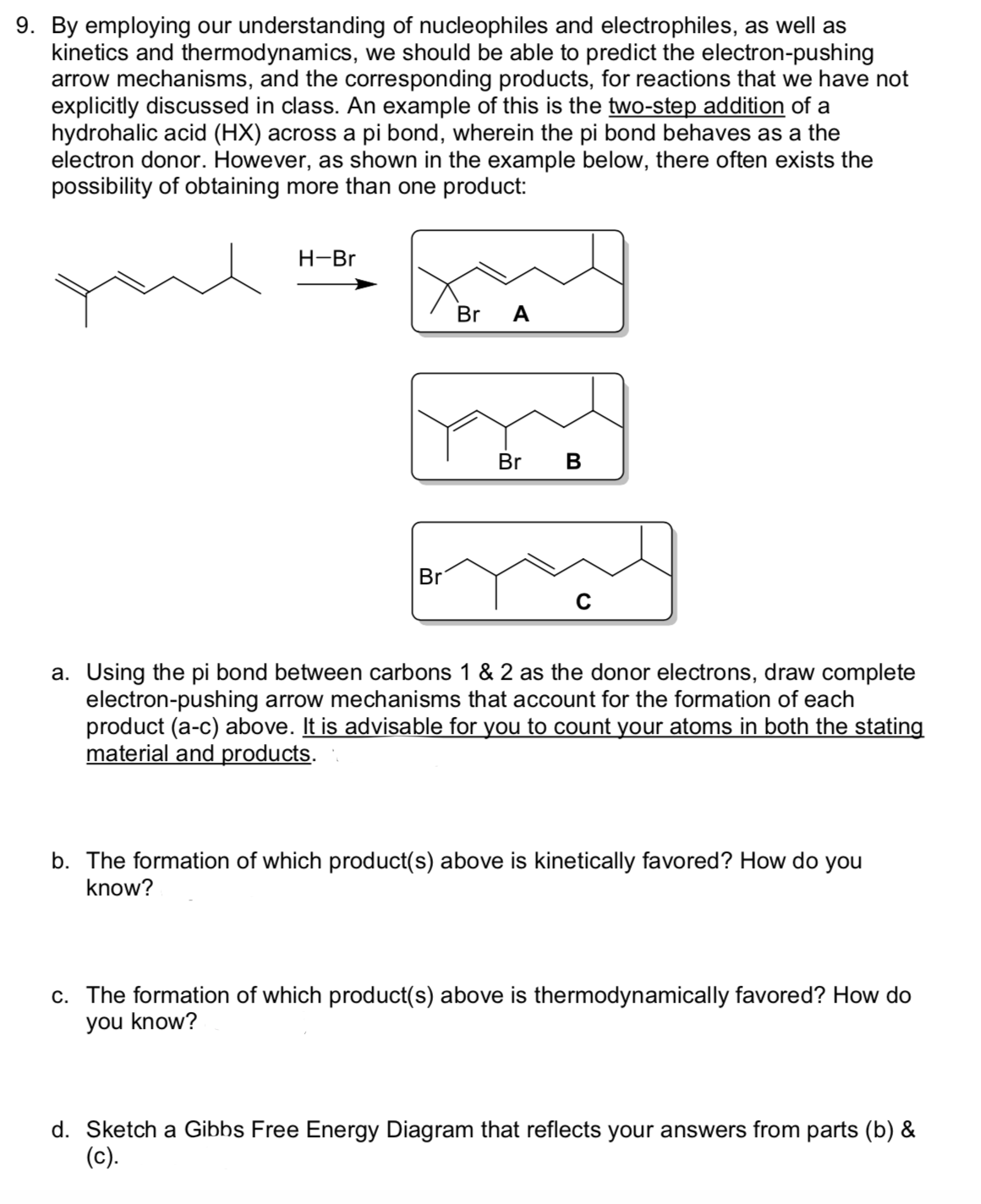
Transcribed Image Text:9. By employing our understanding of nucleophiles and electrophiles, as well as
kinetics and thermodynamics, we should be able to predict the electron-pushing
arrow mechanisms, and the corresponding products, for reactions that we have not
explicitly discussed in class. An example of this is the two-step addition of a
hydrohalic acid (HX) across a pi bond, wherein the pi bond behaves as a the
electron donor. However, as shown in the example below, there often exists the
possibility of obtaining more than one product:
H-Br
Br
A
Br B
Br
a. Using the pi bond between carbons 1 & 2 as the donor electrons, draw complete
electron-pushing arrow mechanisms that account for the formation of each
product (a-c) above. It is advisable for you to count your atoms in both the stating
material and products.
b. The formation of which product(s) above is kinetically favored? How do you
know?
c. The formation of which product(s) above is thermodynamically favored? How do
you know?
d. Sketch a Gibbs Free Energy Diagram that reflects your answers from parts (b) &
(c).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning