9. Consider the reaction shown below in which the radical cation C loses a methyl radical D to generate a cation E. D (i) Which, if any, of the species will be detected by the mass spectrometer? (ii) What percentage of the M" peak will the M" be for each of the detected species? +1 peak +2 (iii) What percentage of the M' peak will the M" peak be for each of the detected species?
9. Consider the reaction shown below in which the radical cation C loses a methyl radical D to generate a cation E. D (i) Which, if any, of the species will be detected by the mass spectrometer? (ii) What percentage of the M" peak will the M" be for each of the detected species? +1 peak +2 (iii) What percentage of the M' peak will the M" peak be for each of the detected species?
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL2: Mass Spectrometry
Section: Chapter Questions
Problem 3E
Related questions
Question
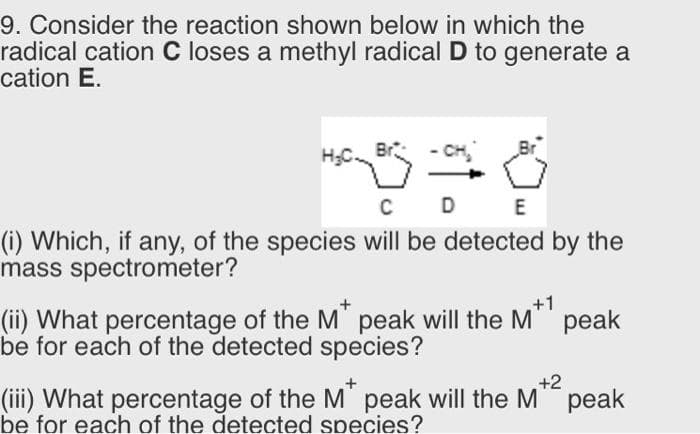
Transcribed Image Text:9. Consider the reaction shown below in which the
radical cation C loses a methyl radical D to generate a
cation E.
- CH
C D
E
(i) Which, if any, of the species will be detected by the
mass spectrometer?
(ii) What percentage of the M" peak will the M"
be for each of the detected species?
рeak
+2
(iii) What percentage of the M' peak will the M" peak
be for each of the detected species?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
