A 10.00 ml. sample of Y(OH)2 was titrated to the stoichiometric point with 14.50 ml of 0.2345 M H2X. The total number of moles of Y(OH)2 is O a. 0.00469 O b. 0.0068 Oc. 0.00034 O d. 0.0034
A 10.00 ml. sample of Y(OH)2 was titrated to the stoichiometric point with 14.50 ml of 0.2345 M H2X. The total number of moles of Y(OH)2 is O a. 0.00469 O b. 0.0068 Oc. 0.00034 O d. 0.0034
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 10P
Related questions
Question
Hi! Please answer B and C.
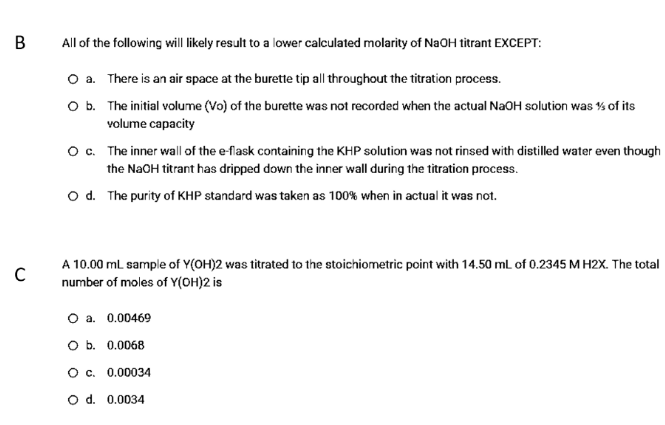
Transcribed Image Text:B
All of the following will likely result to a lower calculated molarity of NAOH titrant EXCEPT:
O a. There is an air space at the burette tip all throughout the titration process.
O b. The initial volume (Vo) of the burette was not recorded when the actual NaOH solution was % of its
volume capacity
o. The inner wall of the e-flask containing the KHP solution was not rinsed with distilled water even though
the NaOH titrant has dripped down the inner wall during the titration process.
o d. The purity of KHP standard was taken as 100% when in actual it was not.
A 10.00 mL sample of Y(OH)2 was titrated to the stoichiometric point with 14.50 mL of 0.2345 M H2X. The total
number of moles of Y(OH)2 is
O a. 0.00469
O b. 0.0068
Oc. 0.00034
O d. 0.0034
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



