A 37.169 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put into a combustion analysis apparatus, yielding 66.214 mg of carbon dioxide and 27.105 mg of water. In another experiment, 39.175 mg of the compound is reacted with excess oxygen to produce 16.93 mg of sulfur dioxide. Add subscripts to the formula provided to correctly identify the empirical formula of this compound. Do not change the order of the elements.
A 37.169 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put into a combustion analysis apparatus, yielding 66.214 mg of carbon dioxide and 27.105 mg of water. In another experiment, 39.175 mg of the compound is reacted with excess oxygen to produce 16.93 mg of sulfur dioxide. Add subscripts to the formula provided to correctly identify the empirical formula of this compound. Do not change the order of the elements.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 2.DCP
Related questions
Question
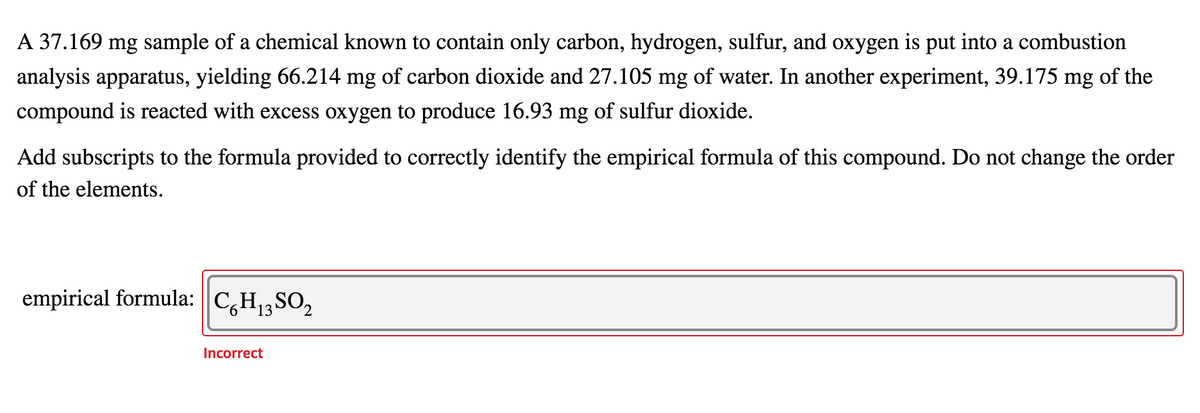
Transcribed Image Text:A 37.169 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put into a combustion
analysis apparatus, yielding 66.214 mg of carbon dioxide and 27.105 mg of water. In another experiment, 39.175 mg of the
compound is reacted with excess oxygen to produce 16.93 mg of sulfur dioxide.
Add subscripts to the formula provided to correctly identify the empirical formula of this compound. Do not change the order
of the elements.
empirical formula: || C,H13 SO2
Incorrect
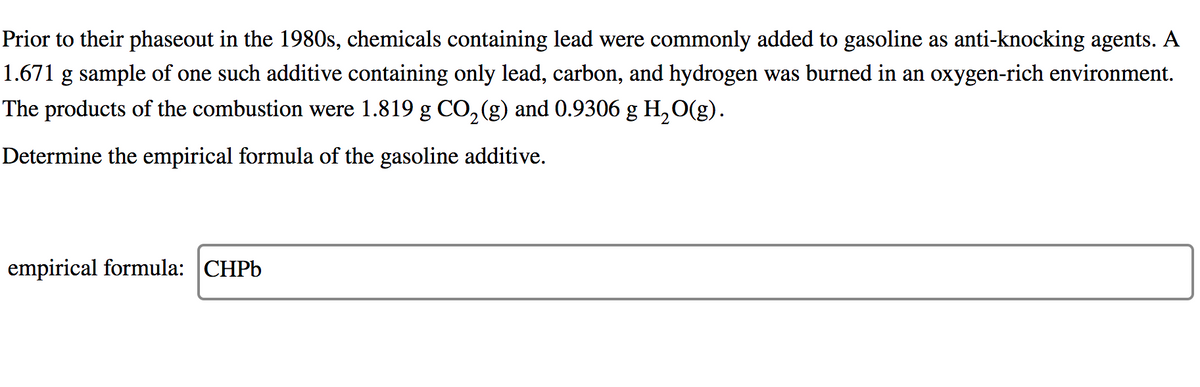
Transcribed Image Text:Prior to their phaseout in the 1980s, chemicals containing lead were commonly added to gasoline as anti-knocking agents. A
1.671 g sample of one such additive containing only lead, carbon, and hydrogen was burned in an oxygen-rich environment.
The products of the combustion were 1.819 g CO,(g) and 0.9306 g H,O(g).
Determine the empirical formula of the gasoline additive.
empirical formula: CHPB
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning