A 6.00 L tank at 29.9 °C is filled with 6.33 g of sulfur tetrafluoride gas and 12.3 g of sulfur hexafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. mole fraction: dlo sulfur tetrafluoride partial pressure: atm mole fraction: sulfur hexafluoride partial pressure: atm Total pressure in tank: atm
A 6.00 L tank at 29.9 °C is filled with 6.33 g of sulfur tetrafluoride gas and 12.3 g of sulfur hexafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. mole fraction: dlo sulfur tetrafluoride partial pressure: atm mole fraction: sulfur hexafluoride partial pressure: atm Total pressure in tank: atm
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.61PAE: 61 As one step in its purification, nickel metal reacts with carbon monoxide to form a compound...
Related questions
Question
100%
Please see image
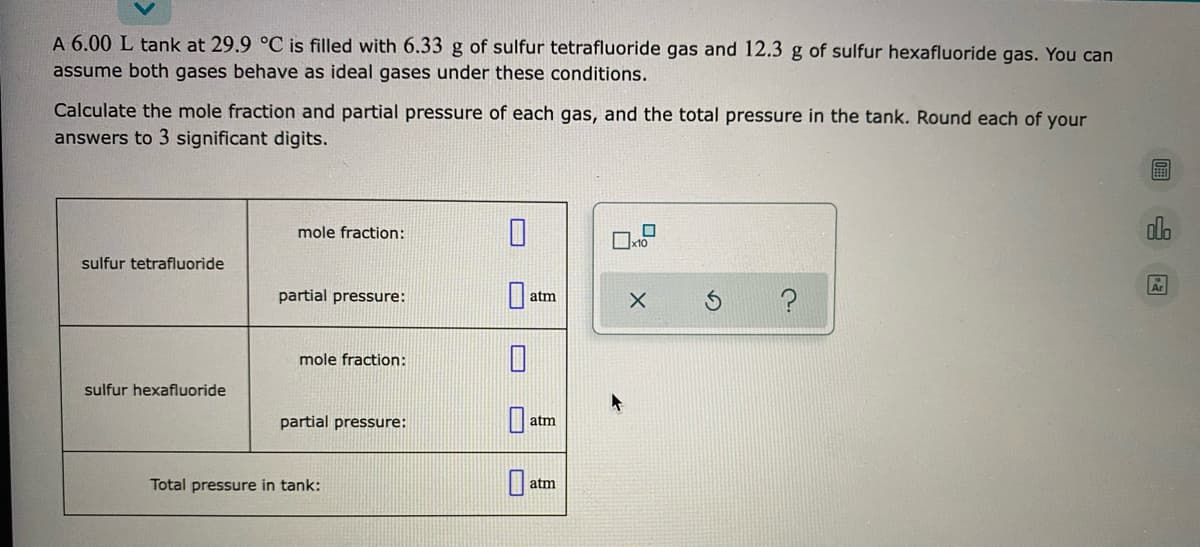
Transcribed Image Text:A 6.00 L tank at 29.9 °C is filled with 6.33 g of sulfur tetrafluoride gas and 12.3 g of sulfur hexafluoride gas. You can
assume both gases behave as ideal gases under these conditions.
Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your
answers to 3 significant digits.
alo
mole fraction:
sulfur tetrafluoride
partial pressure:
atm
mole fraction:
sulfur hexafluoride
partial pressure:
atm
Total pressure in tank:
atm
山园
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning