A 70.0-kg person had a quarter-pound cheeseburger, French fries, and a chocolate shake. Protein (g) Carbohydrate (g) Item Fat (g) 40 Cheeseburger 46 47 French fries 47 16 4 10 Chocolate shake 76 10. a. Calculate the total kilocalories for each food type in this meal b. Determine the total kilocalories for the meal Activity Energy (kcal/h) Energy (kJ/h) Sleeping 60 250 420 Sitting 100 c. Determine the number of hours of sleep needed to burn off the kilocalories in this meal.
A 70.0-kg person had a quarter-pound cheeseburger, French fries, and a chocolate shake. Protein (g) Carbohydrate (g) Item Fat (g) 40 Cheeseburger 46 47 French fries 47 16 4 10 Chocolate shake 76 10. a. Calculate the total kilocalories for each food type in this meal b. Determine the total kilocalories for the meal Activity Energy (kcal/h) Energy (kJ/h) Sleeping 60 250 420 Sitting 100 c. Determine the number of hours of sleep needed to burn off the kilocalories in this meal.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 36E: A pint of premium ice cream can contain 1100 Calories. What mass of fat, in grams and pounds, must...
Related questions
Question
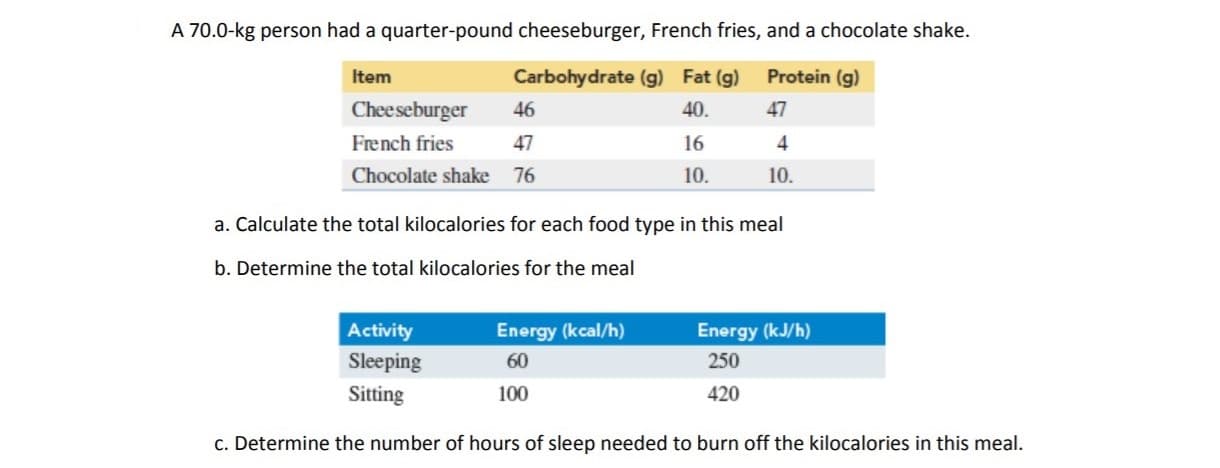
Transcribed Image Text:A 70.0-kg person had a quarter-pound cheeseburger, French fries, and a chocolate shake.
Protein (g)
Carbohydrate (g)
Item
Fat (g)
40
Cheeseburger
46
47
French fries
47
16
4
10
Chocolate shake
76
10.
a. Calculate the total kilocalories for each food type in this meal
b. Determine the total kilocalories for the meal
Activity
Energy (kcal/h)
Energy (kJ/h)
Sleeping
60
250
420
Sitting
100
c. Determine the number of hours of sleep needed to burn off the kilocalories in this meal.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 5 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning