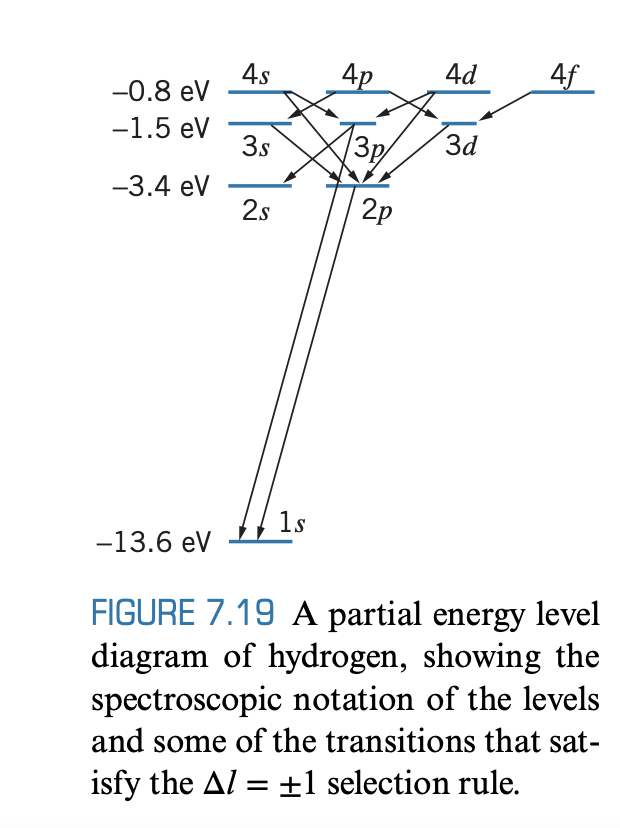
(a) A hydrogen atom is in an excited 5g state, from which it makes a series of transitions by emitting photons, ending in the 1s state. Show, on a diagram similar to Figure 7.19, the sequence of transitions that can occur. (b) Repeat part (a) if the atom begins in the 5d state.
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
-
(a) A hydrogen atom is in an excited 5g state, from which it makes a series of transitions by emitting photons,
ending in the 1s state. Show, on a diagram similar to Figure 7.19, the sequence of transitions that can occur. (b) Repeat part (a) if the atom begins in the 5d state.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images







