Task 1: Electrochemical cell (Galvanic Cell) – Lead nitrate/Lead and Iron(II) nitrate/lron A dark grey electrode made of lead metal is put into a beaker with a colourless solution of 1.0mol/L Lead (II) nitrate. In a separate beaker, another grey electrode made from iron metal is put into a solution of 1.0 mol/L iron (II) nitrate. The two electrodes are connected with a wire and the two solutions are connected with a salt
Task 1: Electrochemical cell (Galvanic Cell) – Lead nitrate/Lead and Iron(II) nitrate/lron A dark grey electrode made of lead metal is put into a beaker with a colourless solution of 1.0mol/L Lead (II) nitrate. In a separate beaker, another grey electrode made from iron metal is put into a solution of 1.0 mol/L iron (II) nitrate. The two electrodes are connected with a wire and the two solutions are connected with a salt
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 76AP
Related questions
Question
Hi there, I really need this chemistry homework to be answered please help me with that I will be really appreciated it. However, I have already done one of them and need this one but I don't know how to do this. Please by looking at my work on the first image complete the second image. I very much need this please help me.
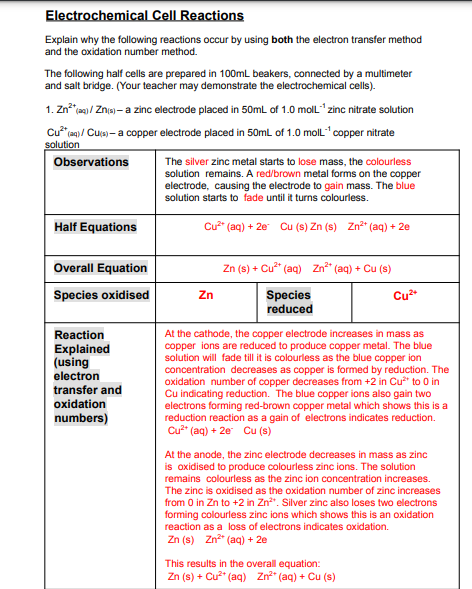
Transcribed Image Text:Electrochemical Cell Reactions
Explain why the following reactions occur by using both the electron transfer method
and the oxidation number method.
The following half cells are prepared in 100mL beakers, connected by a multimeter
and salt bridge. (Your teacher may demonstrate the electrochemical cells).
1. Zn a / Zna - a zinc electrode placed in 50mL of 1.0 moll" zinc nitrate solution
Cu* en)/ Cua – a copper electrode placed in 50mL of 1.0 moll" copper nitrate
solution
Observations
The silver zinc metal starts to lose mass, the colourless
solution remains. A red/brown metal forms on the copper
electrode, causing the electrode to gain mass. The blue
solution starts to fade until it turns colourless.
Half Equations
Cu (aq) + 2e Cu (s) Zn (s) Zn*" (aq) + 2e
Overall Equation
Zn (s) + Cu* (aq) Zn" (aq) + Cu (s)
Species oxidised
Zn
Species
reduced
Cu*
Reaction
Explained
(using
electron
transfer and
oxidation
At the cathode, the copper electrode increases in mass as
copper ions are reduced to produce copper metal. The blue
solution will fade till it is colourless as the blue copper ion
concentration decreases as copper is formed by reduction. The
oxidation number of copper decreases from +2 in Cu* to 0 in
Cu indicating reduction. The blue copper ions also gain two
electrons forming red-brown copper metal which shows this is a
reduction reaction as a gain of electrons indicates reduction.
Cu" (aq) + 2e Cu (s)
numbers)
At the anode, the zinc electrode decreases in mass as zinc
is oxidised to produce colourless zinc ions. The solution
remains colourless as the zinc ion concentration increases.
The zinc is oxidised as the oxidation number of zinc increases
from 0 in Zn to +2 in Zn". Silver zinc also loses two electrons
forming colourless zinc ions which shows this is an oxidation
reaction as a loss of electrons indicates oxidation.
Zn (s) Zn" (aq) + 2e
This results in the overall equation:
Zn (s) + Cu?" (aq) Zn" (aq) + Cu (s)

Transcribed Image Text:Task 1: Electrochemical cell (Galvanic Cell) – Lead nitrate/Lead and
Iron(II) nitrate/Iron
A dark grey electrode made of lead metal is put into a beaker with a colourless
solution of 1.0mol/L Lead (II) nitrate. In a separate beaker, another grey electrode
made from iron metal is put into a solution of 1.0 mol/L iron (II) nitrate. The two
electrodes are connected with a wire and the two solutions are connected with a salt
bridge made from filter paper soaked in KNO, solution. Over time, the mass of the
lead electrode increases but the mass of the iron electrode decreases. The very
pale green iron (II) nitrate solution becomes slightly darker green, while the lead
nitrate solution remains colourless.
V
Electrode: Pb
Electrod
Fe
KNO,
Solutian
Pb(NO,
Fe(NO,
3)2
3)2
Half equations and overall equation:
Species Oxidised:
Species Reduced:
Explain chemistry involved in electrochemical cell using electron transfer and
oxidation numbers. In your discussion you must:
• Link the observations made at each electrode to the species involved.
• Link half equations for the reaction occurring at the anode and cathode and
identify these as either oxidation or reduction.
• Use standard reduction potentials to calculate the cell potential and predict
spontaneity.
• Link the spontaneity to the relative strengths of the oxidant/ reductant
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning