A certain metal M forms a soluble nitrate salt M(NO,) Suppose the left half cell of a galvanic cell apparatus Is filled with a 3.00 M solution of M{NO,), and 3 the right half cell with a 3.00 mM solution of the same substance. Electrodes made of M are dipped Into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 25.0 °C. O left Which electrode will be positive? O right What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.
A certain metal M forms a soluble nitrate salt M(NO,) Suppose the left half cell of a galvanic cell apparatus Is filled with a 3.00 M solution of M{NO,), and 3 the right half cell with a 3.00 mM solution of the same substance. Electrodes made of M are dipped Into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 25.0 °C. O left Which electrode will be positive? O right What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 22E: The mass of three different metal electrodes, each from a different galvanic cell, were determined...
Related questions
Question
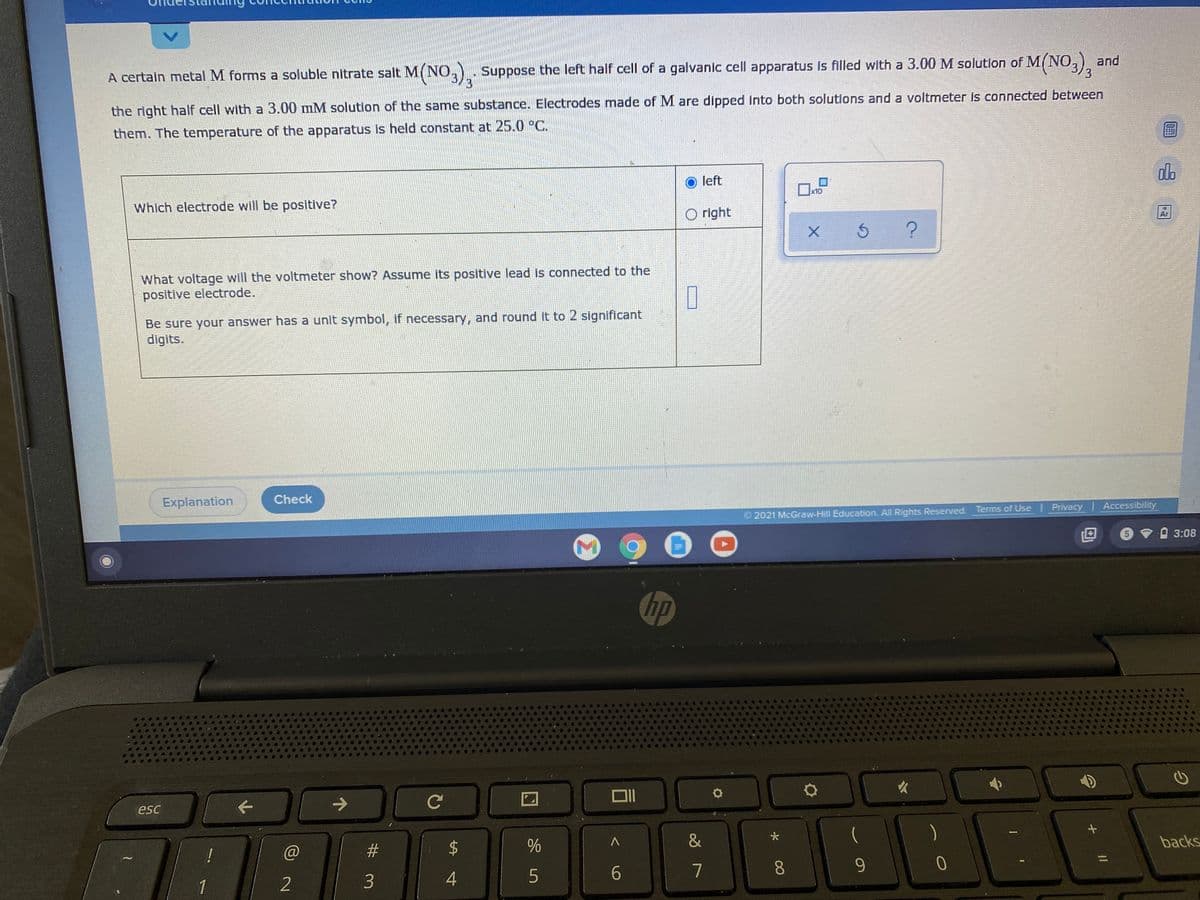
Transcribed Image Text:A certaln metal M forms a soluble nitrate salt M(NO,).
Suppose the left half cell of a galvanic cell apparatus is filled with a 3.00 M solution of M(NO,).
and
the right half cell with a 3.00 mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between
them. The temperature of the apparatus is held constant at 25.0 °C.
O left
do
Which electrode will be positive?
x10
O right
Ar
What voltage will the voltmeter show? Assume its positive lead is connected to the
positive electrode.
Be sure your answer has a unit symbol, if necessary, and round it to 2 significant
digits.
Explanation
Check
2021 McGraw-Hill Education. Ali Rights Reserved. Terms of Use | Privacy Accessibility
O 3:08
hp
esc
!
#3
&
backs
1
2
3
5
6.
7
8.
9.
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
