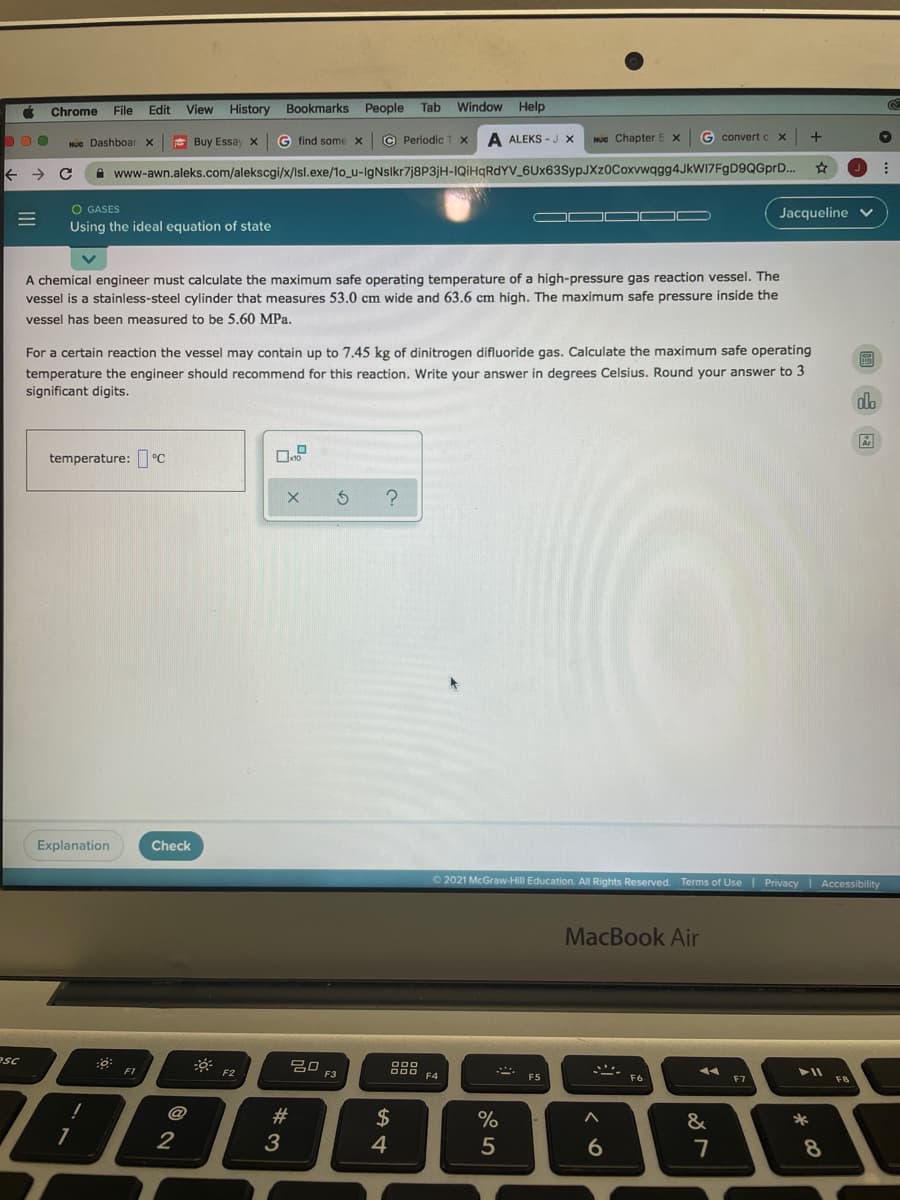
A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 53.0 cm wide and 63.6 cm high. The maximum safe pressure inside the vessel has been measured to be 5.60 MPa. For a certain reaction the vessel may contain up to 7.45 kg of dinitrogen difluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to 3 significant digits. dlo temperature: °C
A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The vessel is a stainless-steel cylinder that measures 53.0 cm wide and 63.6 cm high. The maximum safe pressure inside the vessel has been measured to be 5.60 MPa. For a certain reaction the vessel may contain up to 7.45 kg of dinitrogen difluoride gas. Calculate the maximum safe operating temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to 3 significant digits. dlo temperature: °C
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 8E: Predict and test the behavior of a particles fired at a plum pudding model atom. Predict the paths...
Related questions
Question
Transcribed Image Text:Chrome
File
Edit
View History
Bookmarks People
Tab
Window Help
He Dashboar x
E Buy Essay x
G find some x
© Periodic T x
A ALEKS - J x
Hue Chapter 5 x
G convert c
->
A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQIHQRDYV_6Ux63SypJXz0Coxvwqgg4JkWI7FgD9QGprD..
O GASES
Jacqueline v
Using the ideal equation of state
A chemical engineer must calculate the maximum safe operating temperature of a high-pressure gas reaction vessel. The
vessel is a stainless-steel cylinder that measures 53.0 cm wide and 63.6 cm high. The maximum safe pressure inside the
vessel has been measured to be 5.60 MPa.
For a certain reaction the vessel may contain up to 7.45 kg of dinitrogen difluoride gas. Calculate the maximum safe operating
temperature the engineer should recommend for this reaction. Write your answer in degrees Celsius. Round your answer to 3
significant digits.
temperature: °C
Explanation
Check
O2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy Accessibility
MacBook Air
esc
20
F3
F1
F2
F4
F5
F6
F8
@
#
&
*
2
3
5
6.
7
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning