A chemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. He has a 0.1437 M standard sodium hydroxide solution. He takes a 25.00 mL sample of the original acid solution and dilutes it to 250.0 mL. Then, he takes a 10.00 mL sample of the dilute acid solution and titrates it with the standard solution. The endpoint was reached after the addition of 18.80 mL of the standard solution. What is the concentration of the original sulfuric acid solution? concentration: M
A chemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. He has a 0.1437 M standard sodium hydroxide solution. He takes a 25.00 mL sample of the original acid solution and dilutes it to 250.0 mL. Then, he takes a 10.00 mL sample of the dilute acid solution and titrates it with the standard solution. The endpoint was reached after the addition of 18.80 mL of the standard solution. What is the concentration of the original sulfuric acid solution? concentration: M
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 84E: The concentration of a certain sodium hydroxide solution was determined by using the solution to...
Related questions
Question
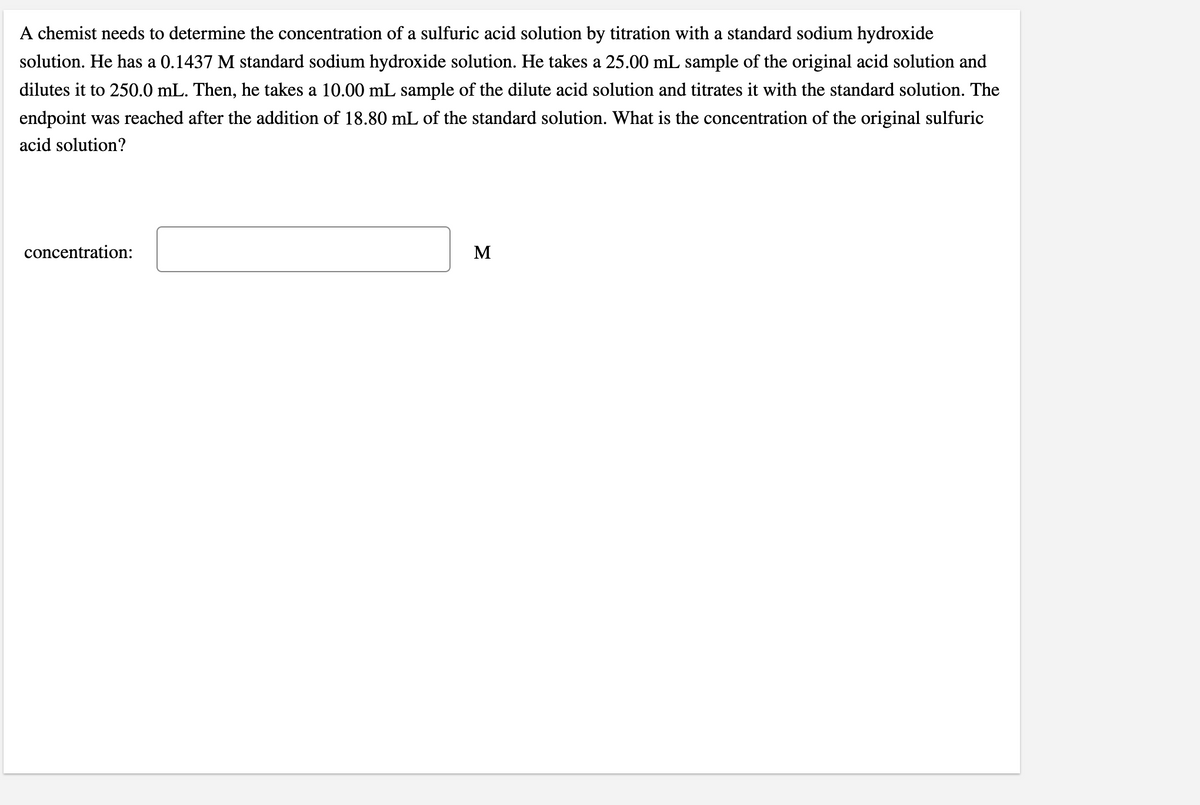
Transcribed Image Text:A chemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide
solution. He has a 0.1437 M standard sodium hydroxide solution. He takes a 25.00 mL sample of the original acid solution and
dilutes it to 250.0 mL. Then, he takes a 10.00 mL sample of the dilute acid solution and titrates it with the standard solution. The
endpoint was reached after the addition of 18.80 mL of the standard solution. What is the concentration of the original sulfuric
acid solution?
concentration:
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning