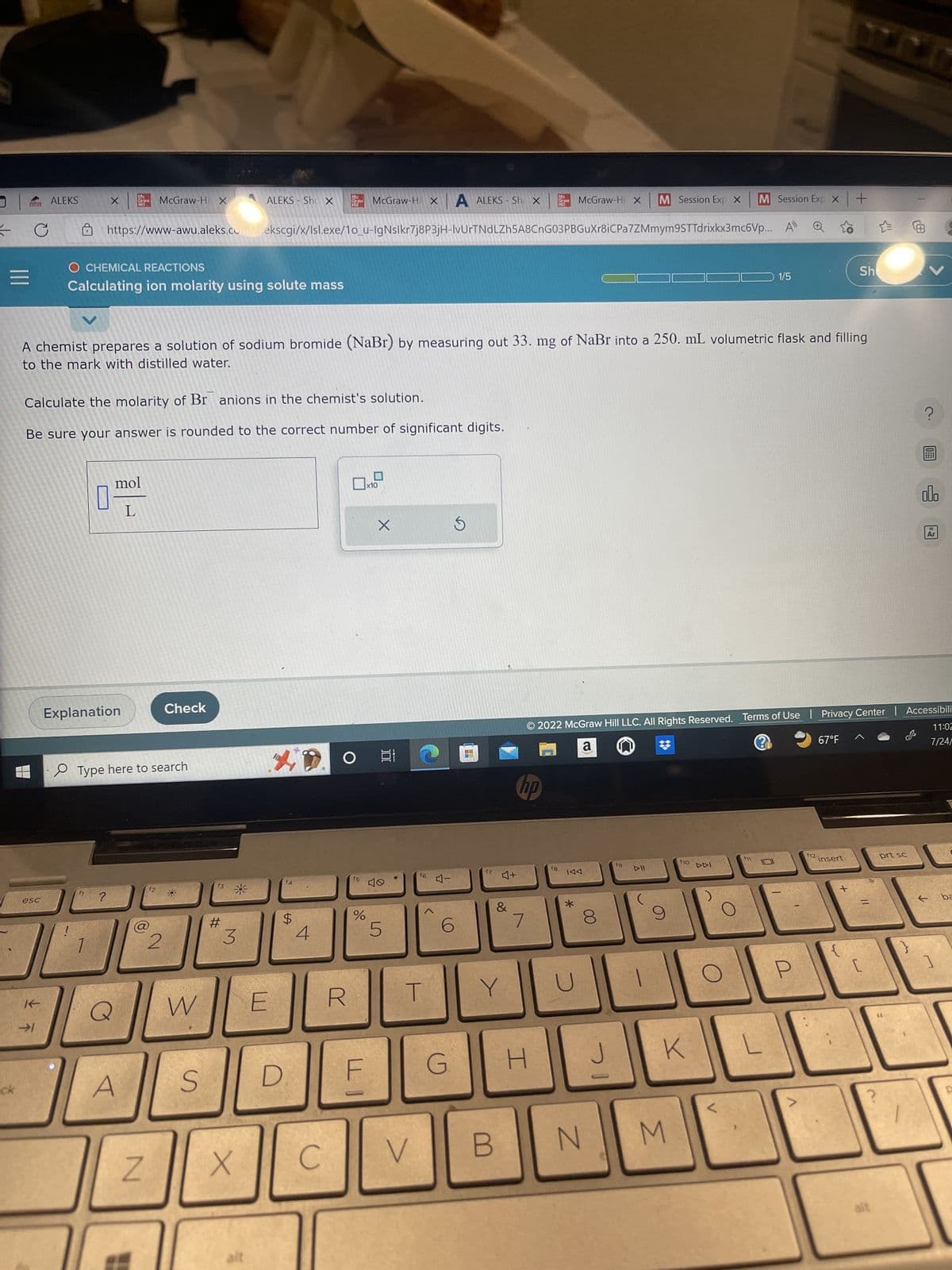
A chemist prepares a solution of sodium bromide (NaBr) by measuring out 33. mg of NaBr into a 250. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits. O mol L x10 X ?圖 ol Ar
A chemist prepares a solution of sodium bromide (NaBr) by measuring out 33. mg of NaBr into a 250. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits. O mol L x10 X ?圖 ol Ar
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter34: Particle Size Determination
Section: Chapter Questions
Problem 34.10QAP
Related questions
Question
Make sure has correct significant di
Transcribed Image Text:J
=
ck
PR
esc
ALEKS
K-
→1
McGraw-Hi X M Session Exp X M Session Exp X
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym9STTdrixkx3mc6Vp... A
X
O CHEMICAL REACTIONS
Calculating ion molarity using solute mass
Calculate the molarity of Br anions in the chemist's solution.
Be sure your answer is rounded to the correct number of significant digits.
1 ?
Mc
Graw McGraw-Hi X
Explanation
mol
L
A chemist prepares a solution of sodium bromide (NaBr) by measuring out 33. mg of NaBr into a 250. mL volumetric flask and filling
to the mark with distilled water.
Type here to search
Q
A
Z
12
Check
2
W
S
ALEKS- Shu X
#
3
X
E
4
D
f4
$
Mc
McGraw-Hi X
Graw
Hill
4
C
O
f5
%
LI
F
x10
X
100
10
5
f6
T
V
4-
A ALEKS-Sh x
6
G
S
4
HH
17
4+
B
80
&
Y
hp
1
H
Graw
f8
IAA
*
8
J
2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibili
a
2
11:02
7/24/
N
기
fg
DII
f10
K
M
DDI
O
1/5
L
0
2
>
f12
+
67°F
Sh
insert
C
alt
?
prt sc
10
alo
18
Ar
1
ba
P
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning