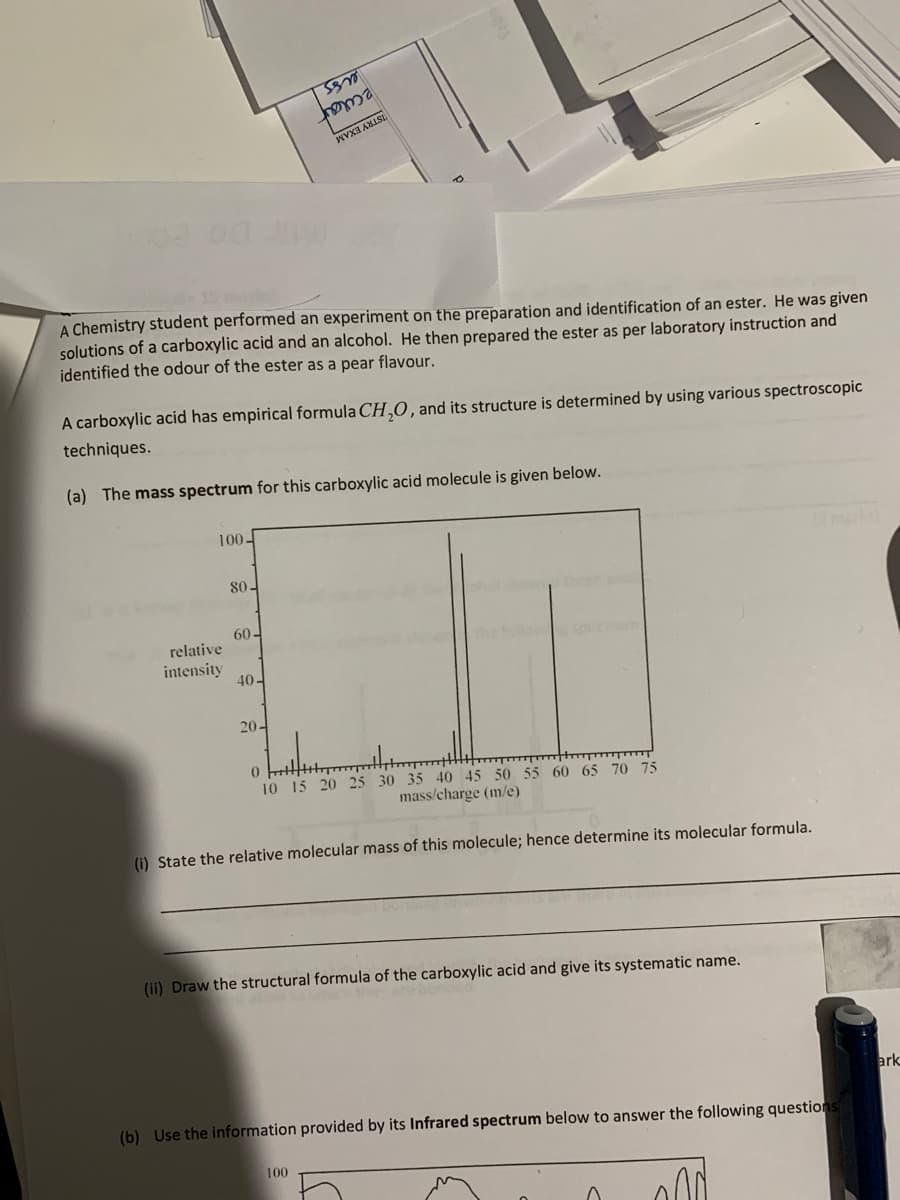
A Chemistry solutions of a carboxylic acid and an alcohol. He then prepared the ester as per laboratory instrü identified the odour of the ester as a pear flavour. A carboxylic acid has empirical formula CH,O, and its structure is determined by using various spectroscopic techniques. (a) The mass spectrum for this carboxylic acid molecule is given below. 100- 80- 60- relative intensity 40- 20- 10 15 20 25 30 35 40 45 50 55 60 65 70 75 mass/charge (m/e) (1) State the relative molecular mass of this molecule; hence determine its molecular formula. (ii) Draw the structural formula of the carboxylic acid and give its systematic name.
A Chemistry solutions of a carboxylic acid and an alcohol. He then prepared the ester as per laboratory instrü identified the odour of the ester as a pear flavour. A carboxylic acid has empirical formula CH,O, and its structure is determined by using various spectroscopic techniques. (a) The mass spectrum for this carboxylic acid molecule is given below. 100- 80- 60- relative intensity 40- 20- 10 15 20 25 30 35 40 45 50 55 60 65 70 75 mass/charge (m/e) (1) State the relative molecular mass of this molecule; hence determine its molecular formula. (ii) Draw the structural formula of the carboxylic acid and give its systematic name.
Chapter13: Isolation Of Eugenol From Clov
Section: Chapter Questions
Problem 9Q
Related questions
Question
Transcribed Image Text:2clar
ISTRY EXAM
A Chemistry student performed an experiment on the preparation and identification of an ester. He was given
solutions of a carboxylic acid and an alcohol. He then prepared the ester as per laboratory instruction and
identified the odour of the ester as a pear flavour.
A carboxylic acid has empirical formula CH,O, and its structure is determined by using various spectroscopic
techniques.
(a) The mass spectrum for this carboxylic acid molecule is given below.
100-
80-
60-
relative
intensity
40-
20-
10 15 20 25 30 35 40 45 50 55 60 65 70 75
mass/charge (m/e)
(i) State the relative molecular mass of this molecule; hence determine its molecular formula.
(ii) Draw the structural formula of the carboxylic acid and give its systematic name.
ark
(b) Use the information provided by its Infrared spectrum below to answer the following questions
100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning
