A chemlstry graduate student Is glven 300. mL of a 1.50M trimethylamine ((CH, N solutlon. Trimethylamine Is a weak base with K, =7.4 x 10. What (CH3),NHBr should the student dissolve In the (CH,),N solution to turn It Into a buffer with pH = 11.107 mass of You may assume that the volume of the solution doesn't change when the (CH,) NHB1 is dissolved in it. Be sure your answer has a unit symbol, and round It to 2 significant digits.
A chemlstry graduate student Is glven 300. mL of a 1.50M trimethylamine ((CH, N solutlon. Trimethylamine Is a weak base with K, =7.4 x 10. What (CH3),NHBr should the student dissolve In the (CH,),N solution to turn It Into a buffer with pH = 11.107 mass of You may assume that the volume of the solution doesn't change when the (CH,) NHB1 is dissolved in it. Be sure your answer has a unit symbol, and round It to 2 significant digits.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 84E: A saturated solution of a slightly soluble electrolyte in contact with some of the solid electrolyte...
Related questions
Question
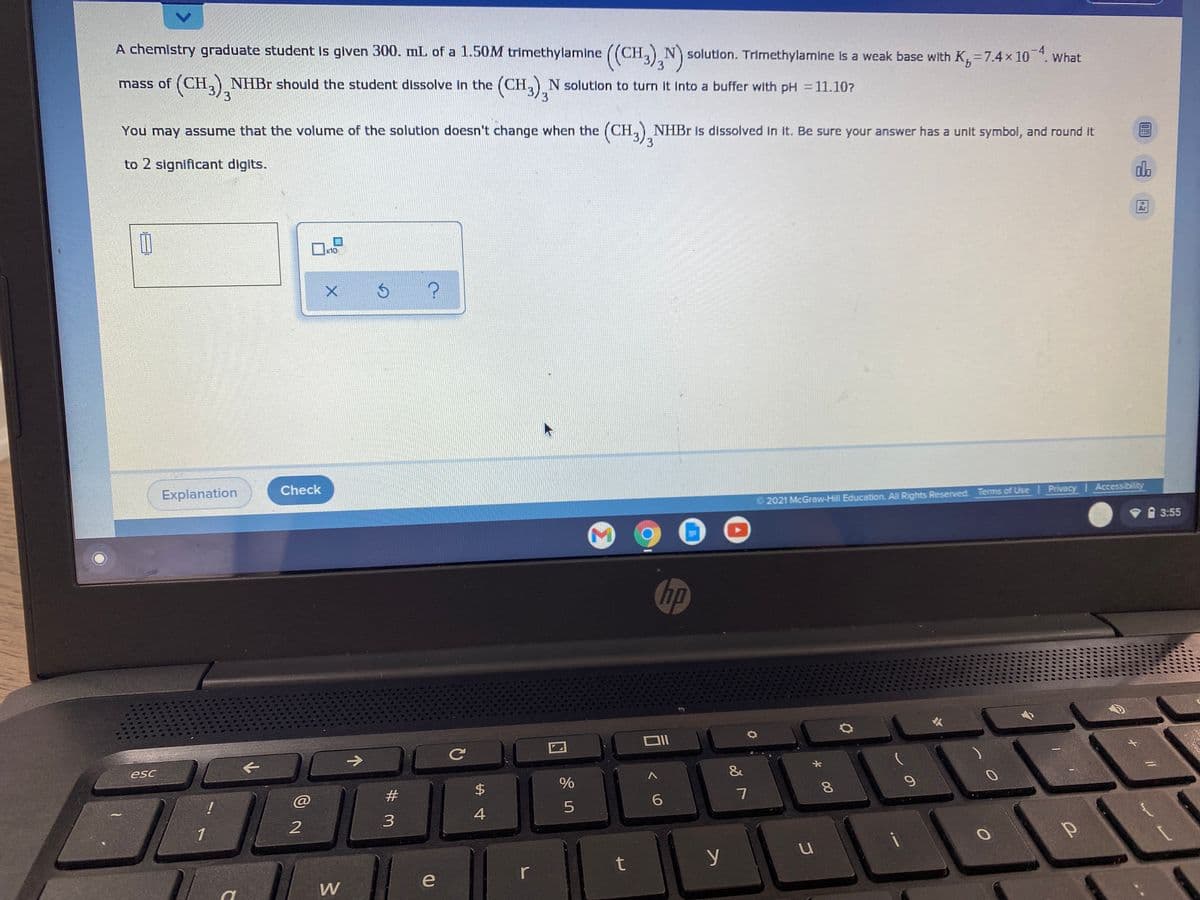
Transcribed Image Text:A chemistry graduate student Is glven 300. mL of a 1.50M trimethylamine ((CH, N solutlon. Trimethylamine Is a weak base with K,=7.4x 10 ". what
3.
mass of (CH,NHBr should the student dissolve in the (CH, N solutlon to turn It Into a buffer with pH = 11.107
(CH),
3
You may assume that the volume of the solution doesn't change when the (CH,) NHBR Is dissolved In it. Be sure your answer has a unit symbol, and round It
3
to 2 significant digits.
do
Ar
x10
Check
Explanation
2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Accessibility
V I 3:55
hp
火
Ce
&
esc
$4
8.
#3
!
4
3
t
y
r
e
W
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co