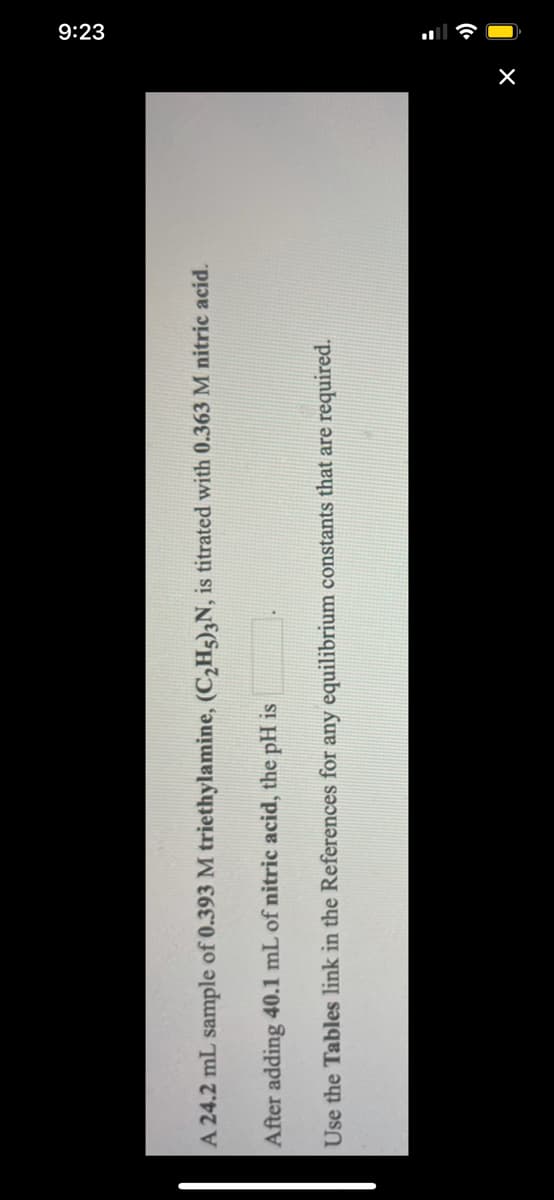
umple of 0.393 M triethylamine, (C,H5),N, is titrated with 0.3 40.1 mL of nitric acid, the pH is es link in the References for any equilibrium constants that are
umple of 0.393 M triethylamine, (C,H5),N, is titrated with 0.3 40.1 mL of nitric acid, the pH is es link in the References for any equilibrium constants that are
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.22QAP
Related questions
Question
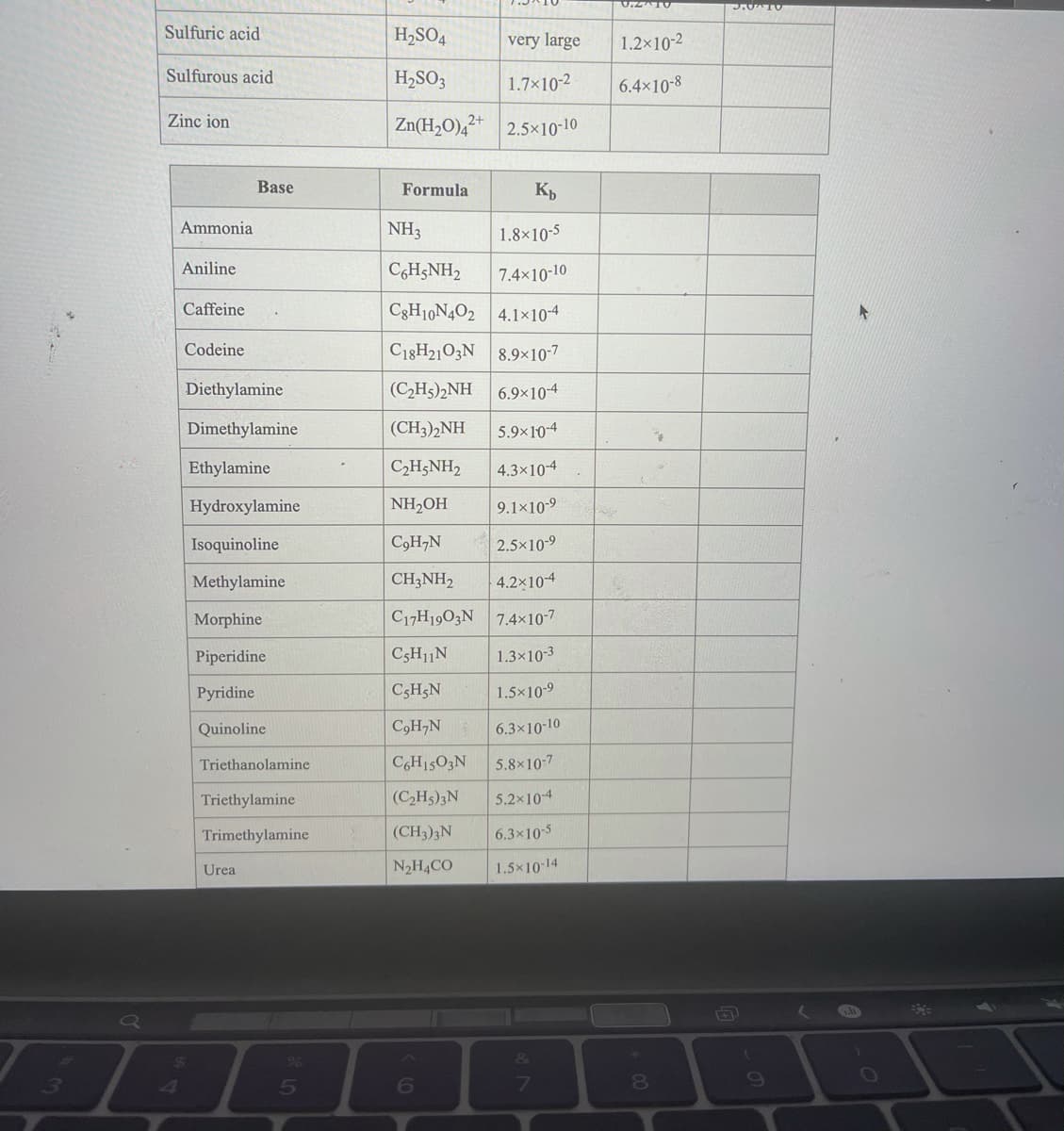
Transcribed Image Text:Sulfuric acid
H,SO4
very large
1.2x10-2
Sulfurous acid
H2SO3
1.7×10-2
6.4×10-8
Zinc ion
Zn(H2O),²+ 2.5x10-10
Base
Formula
Ammonia
NH3
1.8×10-5
Aniline
CgH;NH2
7.4x10-10
Caffeine
C3H10N4O2 4.1×10-4
Codeine
C18H21O3N 8.9×10-7
Diethylamine
(C2H5)2NH
6.9×10-4
Dimethylamine
(CH3)2NH
5.9×10-4
Ethylamine
C,H;NH2
4.3×10-4
Hydroxylamine
NH,OH
9.1×10-9
Isoquinoline
C9H,N
2.5×10-9
Methylamine
CH3NH2
4.2x104
Morphine
C17H1903N 7.4×10-7
Piperidine
C3H11N
1.3×10-3
Pyridine
C5H5N
1.5x10-9
Quinoline
C,H,N
6.3x10-10
Triethanolamine
CGH15O3N
5.8x10-7
Triethylamine
(C,H5)3N
5.2x10-4
Trimethylamine
(CH3)3N
6.3x10-5
Urea
N2H4CO
1.5x10-14
6
80
Transcribed Image Text:9:23
A 24.2 mL sample of 0.393 M triethylamine, (C,Hg),N, is titrated with 0.363 M nitric acid.
After adding 40.1 mL of nitric acid, the pH is
Use the Tables link in the References for any equilibrium constants that are required.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning