Chapter2: Crystallization
Section: Chapter Questions
Problem 3Q
Related questions
Question
100%
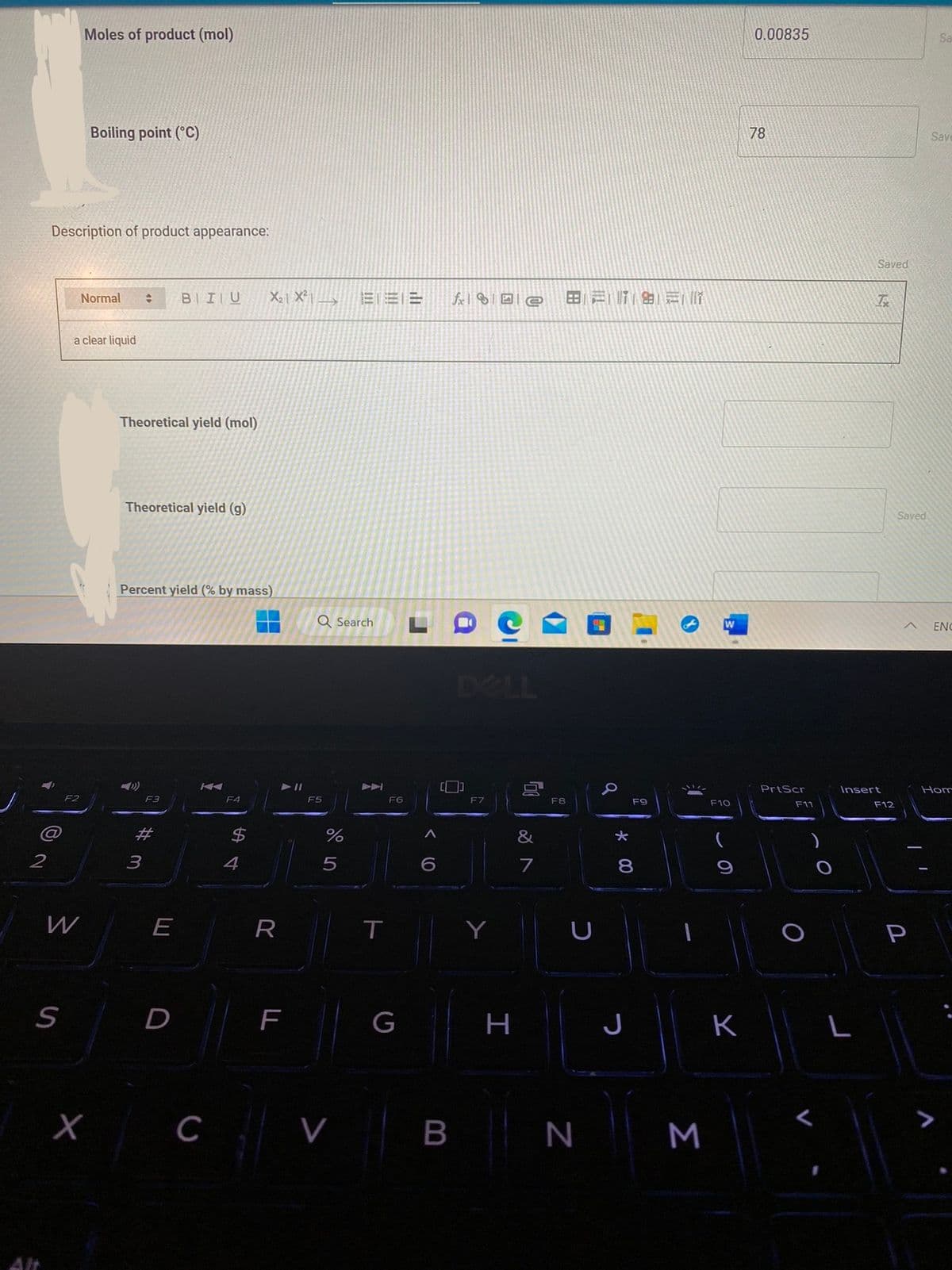
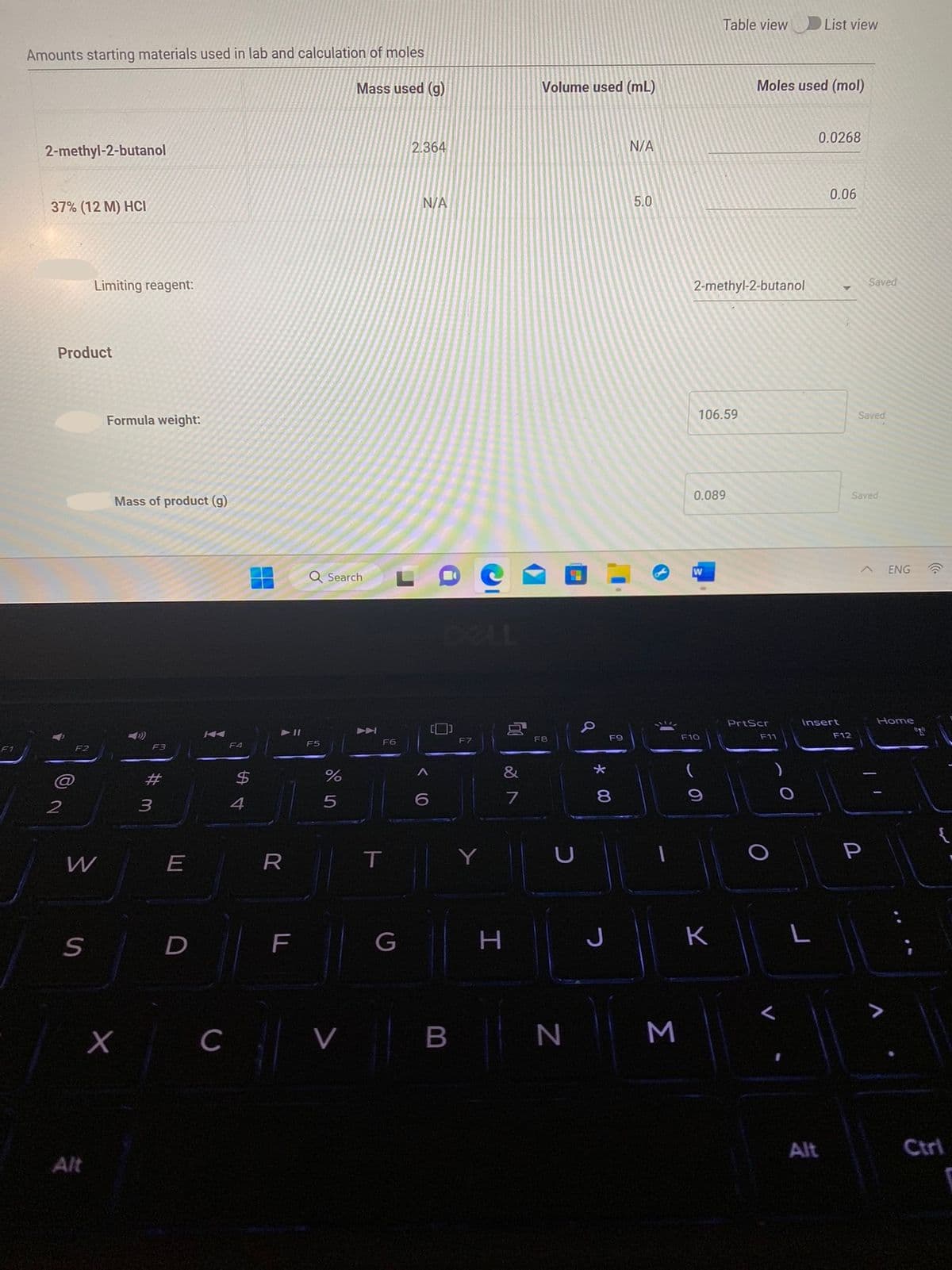
find theoretical yield (g and mol) and percent yield please
Transcribed Image Text:2
Alt
S
Moles of product (mol)
Description of product appearance:
Boiling point (°C)
W
Normal ● BII U X₂1X²1
a clear liquid
X
Theoretical yield (mol)
Theoretical yield (g)
Percent yield (% by mass)
2
F3
#m
3
E
D
Y
C
F4
St
R
F
Q Search
F5
dlo s
%
5
EIES f
V
1
T
F6
G
<
O
B
F7
Y
H
e
&
7
F8
Bi
U
N
a
* 00
F9
8
J
M
W
F10
K
0.00835
78
PrtScr
F11
Saved
L
T
Insert
F12
Saved
P
Sa
Save
ENC
Hom
Transcribed Image Text:F1
Amounts starting materials used in lab and calculation of moles
Mass used (g)
2-methyl-2-butanol
37% (12 M) HCI
Product
F2
Limiting reagent:
W
S
Alt
Formula weight:
Mass of product (g)
X
3
F3
#3
E
D
KA
C
F4
54
N
► 11
R
F
Q Search
F5
olo s
%
V
F6
T
G
2.364
N/A
A
0
B
F7
>
19
&
7
H
Volume used (mL)
F8
U
N
a
* 00
J
F9
N/A
5.0
€1
M
2-methyl-2-butanol
106.59
0.089
W
Table view
F10
の
K
Moles used (mol)
PrtScr
F11
List view
L
0.0268
Insert
Alt
0.06
F12
Saved
Saved
Saved
P
ENG
Home
09
Ctrl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
