A combustion analysis is a technique to find the the empirical formula of compounds. In a combustion analysis experiment, 0.8657 g of an organic.compound containing only carbon, hydrogen, and oxygen is burned in oxygen, forming 1.190 g of CO2 and 0.3248 g of H20. What is the empirical formula of the compound? Write the numbers that you would write in the subscript of the formula in the boxes next to the symbol of the element. C H.
A combustion analysis is a technique to find the the empirical formula of compounds. In a combustion analysis experiment, 0.8657 g of an organic.compound containing only carbon, hydrogen, and oxygen is burned in oxygen, forming 1.190 g of CO2 and 0.3248 g of H20. What is the empirical formula of the compound? Write the numbers that you would write in the subscript of the formula in the boxes next to the symbol of the element. C H.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section4.4: Chemical Equations And Chemical Analysis
Problem 1RC: A 0509-g sample of an unknown organic compound containing C, H, and O was burned in air to give...
Related questions
Question
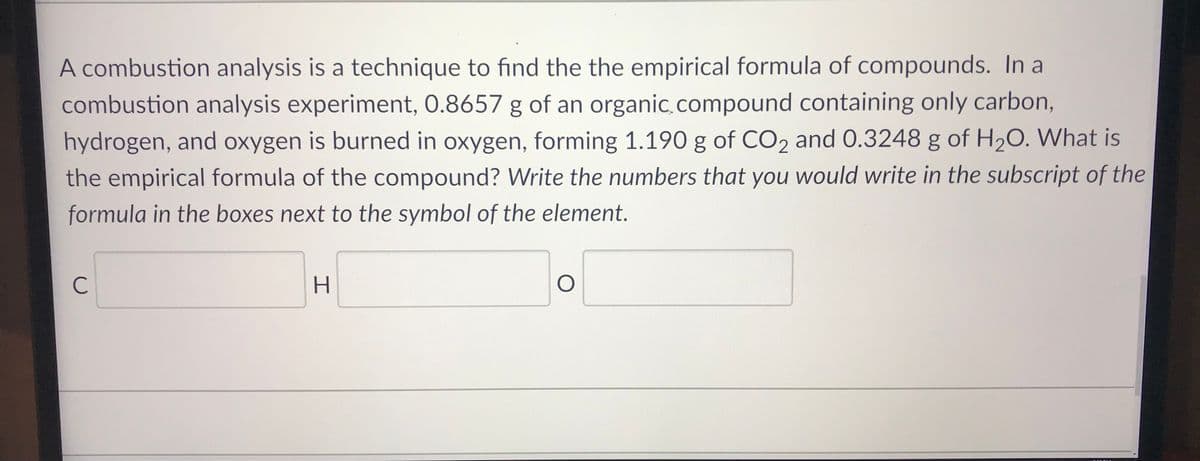
Transcribed Image Text:A combustion analysis is a technique to find the the empirical formula of compounds. In a
combustion analysis experiment, 0.8657 g of an organic.compound containing only carbon,
hydrogen, and oxygen is burned in oxygen, forming 1.190 g of CO2 and 0.3248 g of H2O. What is
the empirical formula of the compound? Write the numbers that you would write in the subscript of the
formula in the boxes next to the symbol of the element.
C
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: Which of the following apply to bonds? Select all the apply
a. Earns gains from dividends
b. Earns…
Q: In 2006, 12.5% of all live births in the United States were to
mothers under 20 years of age. A…
Q: Suppose that, for a certain exam, a teacher grades on a curve.
Frequency
0.1%
2.2%
34.1% 34.1%
2.2%…
Q: Please don't provide handwritten solution ....
Q: Please don't provide handwritten solution ....
Q: Help pls :(
Q: All parts
Q: When you take the Scholastic Assessment Test (SAT), your score is recorded as a percentile score. If…
Q: Please don't providehandwritten solution ....
Q: In a secret government facility, a biological weapons test goes wrong, turning 10 test subjects
into…
Q: Part a,b
Q: Find the three measures of central tendency (the mean, median, and mode). (If an answer does not…
Q: Parts a,b,c
Q: Pleasedon't provide handwritten solution...
Q: Part a,b,c
Q: Hi, can you help me with this PDE?
Q: Hi, can you help me with this PDE?
Q: 2. Consider a simple AM radio consisting of a tunable L-C circuit. The input is the voltage
received…
Q: 1. The vibration of a dental drill is governed by the following differential equation
+0.1+ (106+…
Q: Leafair Rickson Co. empties its
inventory stores every 18.25 days.
The firm orders 534,000 units to…
Q: Please write the answer now