A combustion analysis is performed on a compound that contains only C and H. The analysis produces 7.69 g of CO2 and 3.17g of H20. In addition, it was determined that the molar mass of the compound is 56.107 g/mol. Determine the molecular formula of the compound. Given molar masses: CO2: 44.009 g/mol; H2O: 18.015 g/mol
A combustion analysis is performed on a compound that contains only C and H. The analysis produces 7.69 g of CO2 and 3.17g of H20. In addition, it was determined that the molar mass of the compound is 56.107 g/mol. Determine the molecular formula of the compound. Given molar masses: CO2: 44.009 g/mol; H2O: 18.015 g/mol
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter19: The Chemistry Of The Main-group Elements
Section19.6: A Periodic Perspective: The Main-group Elements
Problem 19.14E
Related questions
Question
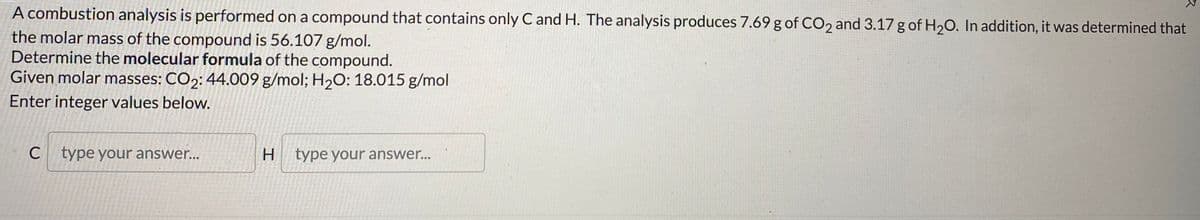
Transcribed Image Text:A combustion analysis is performed on a compound that contains only C and H. The analysis produces 7.69 g of CO2 and 3.17 g of H20. In addition, it was determined that
the molar mass of the compound is 56.107 g/mol.
Determine the molecular formula of the compound.
Given molar masses: CO2: 44.009 g/mol; H2O: 18.015 g/mol
Enter integer values below.
type your answer...
H.
type your answer...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax