A compound has the following % composition data 49 48% Carbon 5.15% Hydrogen 28.87% Nitrogen 16.49% Oxygen The molecular weight is between 180 g/mole and 200 g/mole Determine the empirical and molecular formula
A compound has the following % composition data 49 48% Carbon 5.15% Hydrogen 28.87% Nitrogen 16.49% Oxygen The molecular weight is between 180 g/mole and 200 g/mole Determine the empirical and molecular formula
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter3: Equation, The Mole, And Chemical Formulas
Section: Chapter Questions
Problem 3.85QE: A chemist prepared a compound that she thought had the formula FeI3. When the compound was analyzed,...
Related questions
Question
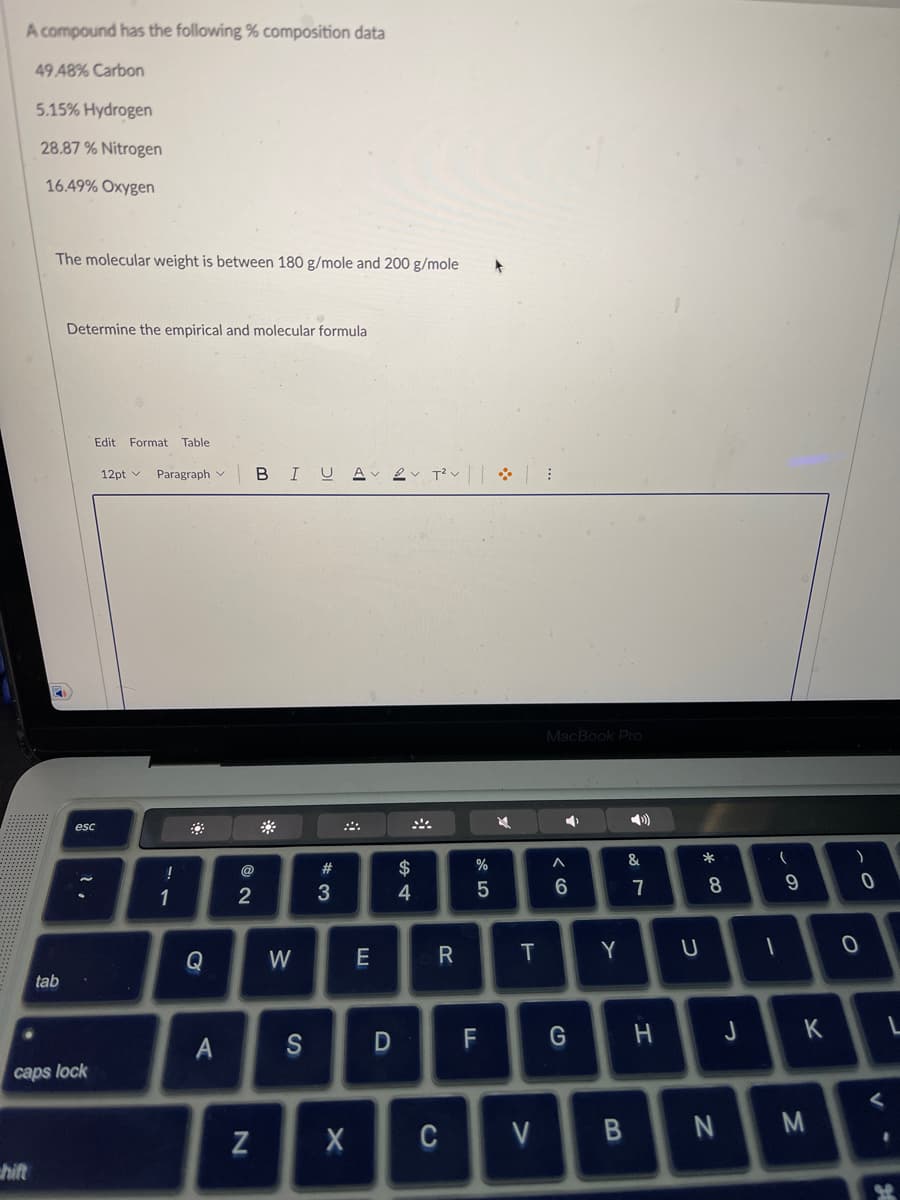
Transcribed Image Text:A compound has the following % composition data
49 48% Carbon
5.15% Hydrogen
28.87% Nitrogen
16.49% Oxygen
hift
The molecular weight is between 180 g/mole and 200 g/mole
tab
Determine the empirical and molecular formula
caps lock
Edit Format Table
esc
12pt v Paragraph
!
1
Q
A
2
N
BIU AT²V
W
#3
E
S D
$
4
4
R
X C
%
5
F
M
T
V
MacBook Pro
A
6
G
Y
B
27
&
U
*00
8
-
N
(
9
H J K
M
)
O-
0
0
<
St
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax




Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning