A cryogenic vacuum pump works by condensing vapors onto some absorbent medium. This is an efficient and clean way to pump a system in a research environment. The term cryo means cold, which indicates that these types of vacuum pumps contain a refrigerant cycle to cool the internal parts. The temperature difference between the inside and outside of a typical cryogenic pump is AT = 312°C. Derive an expression to convert this difference into Fahrenheit and express the answer.
A cryogenic vacuum pump works by condensing vapors onto some absorbent medium. This is an efficient and clean way to pump a system in a research environment. The term cryo means cold, which indicates that these types of vacuum pumps contain a refrigerant cycle to cool the internal parts. The temperature difference between the inside and outside of a typical cryogenic pump is AT = 312°C. Derive an expression to convert this difference into Fahrenheit and express the answer.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 20E: A 45-g aluminum spoon (specific heat 0.88 J/g C) at 24 C is placed in 180 mL (180 g) of coffee at 85...
Related questions
Question
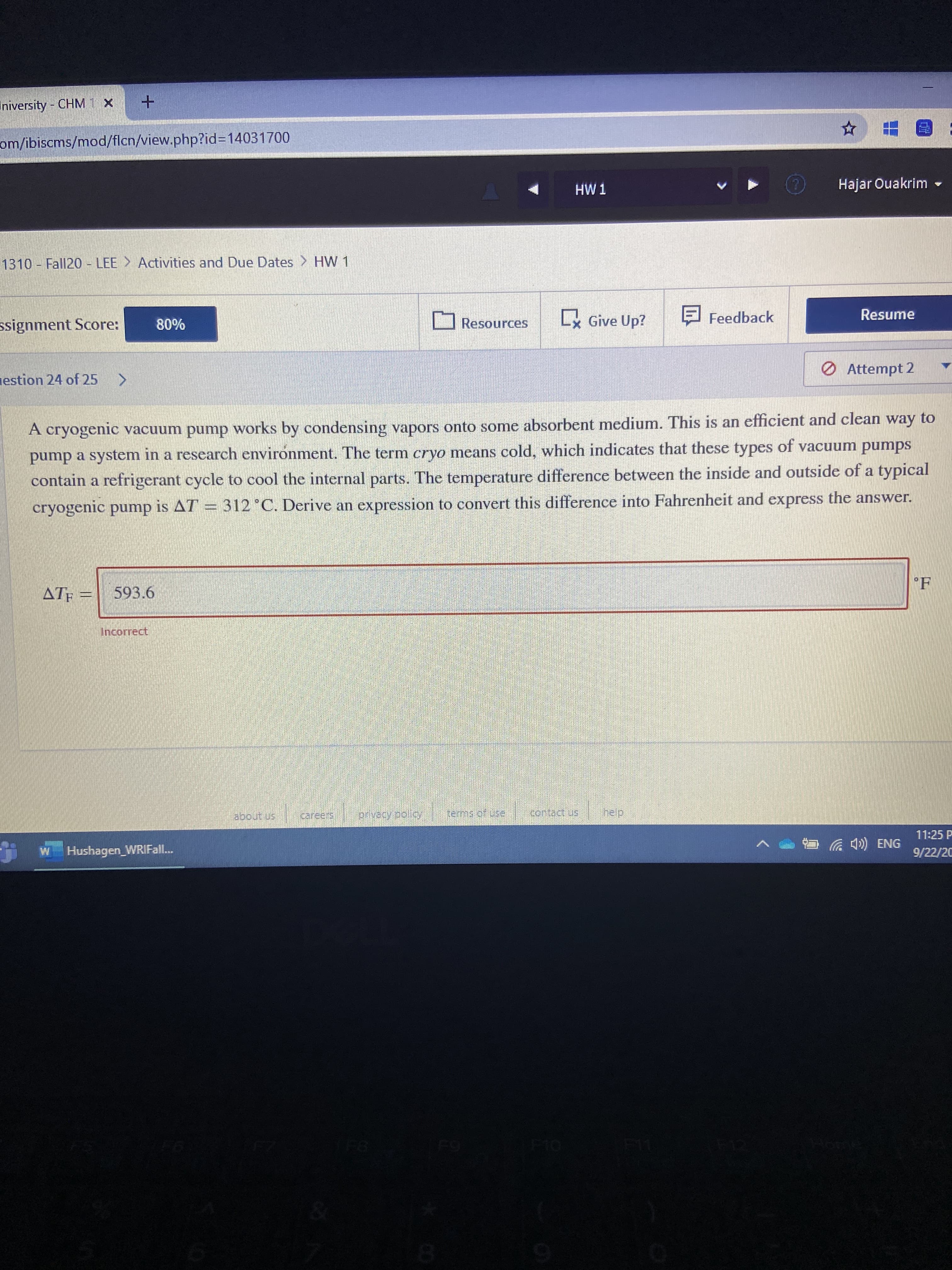
Transcribed Image Text:A cryogenic vacuum pump works by condensing vapors onto some absorbent medium. This is an efficient and clean way to
pump a system in a research environment. The term cryo means cold, which indicates that these types of vacuum pumps
contain a refrigerant cycle to cool the internal parts. The temperature difference between the inside and outside of a typical
cryogenic pump is AT
= 312°C. Derive an expression to convert this difference into Fahrenheit and express the answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning