(a) For refractory ceramic materials, cite two characteristics that improve with and two characteristics that are adversely affected by increasing porosity.
(a) For refractory ceramic materials, cite two characteristics that improve with and two characteristics that are adversely affected by increasing porosity.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter22: Inorganic Materials
Section: Chapter Questions
Problem 7P
Related questions
Question
Transcribed Image Text:Question Seven
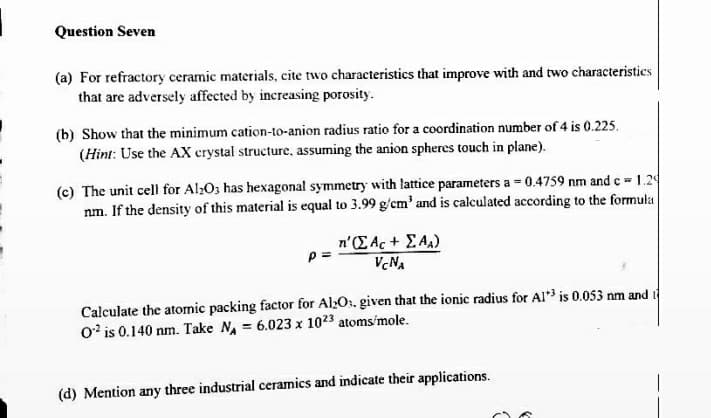
(a) For refractory ceramic materials, cite two characteristics that improve with and two characteristics
that are adversely affected by increasing porosity.
(b) Show that the minimum cation-to-anion radius ratio for a coordination number of 4 is 0.225.
(Hint: Use the AX crystal structure, assuming the anion spheres touch in plane).
(c) The unit cell for Al>O; has hexagonal symmetry with lattice parameters a = 0.4759 nm and c 1.2
nm. If the density of this material is equal to 3.99 g/cm' and is calculated according to the formuka
n'(EAc+ EA)
VcNA
Calculate the atormic packing factor for Al>O1, given that the ionic radius for AI is 0.053 nm and i
0' is 0.140 nm. Take N = 6.023 x 1023 atoms'mole.
(d) Mention any three industrial ceramics and indicate their applications.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,