A heat engine operates on a Carnot cycle. In this cycles, it absorbs 360J of energy while it expands in contact with a reservoir of temperature Th. The engine exhausts 220J as heat as it contracts in contact with a reservoir of temperature Te. 10. How much work does the engine perform during a single cycle? (a) ***140J (b) 220.J (c) 360J (d) 580.J (e) There is not enough information to determine this. 11. If the temperature at which energy is exhauste is Tc = 300K, what is the temperature at which energy was absorbed? (a) 117K (b) 183K (c) 300K (d) ***490K (e) 771K
A heat engine operates on a Carnot cycle. In this cycles, it absorbs 360J of energy while it expands in contact with a reservoir of temperature Th. The engine exhausts 220J as heat as it contracts in contact with a reservoir of temperature Te. 10. How much work does the engine perform during a single cycle? (a) ***140J (b) 220.J (c) 360J (d) 580.J (e) There is not enough information to determine this. 11. If the temperature at which energy is exhauste is Tc = 300K, what is the temperature at which energy was absorbed? (a) 117K (b) 183K (c) 300K (d) ***490K (e) 771K
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
Hi could someone please walk me through why these answers are what they are. Thank you.
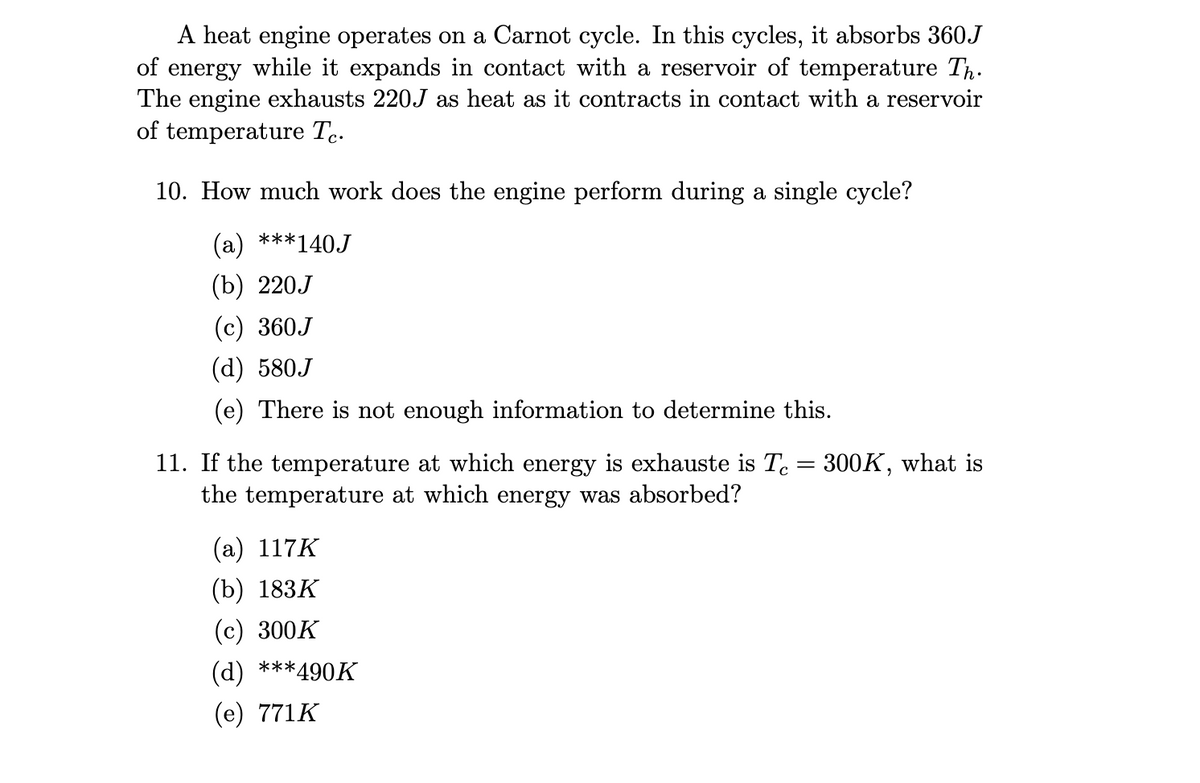
Transcribed Image Text:A heat engine operates on a Carnot cycle. In this cycles, it absorbs 360J
of energy while it expands in contact with a reservoir of temperature Th.
The engine exhausts 220J as heat as it contracts in contact with a reservoir
of temperature Tc.
10. How much work does the engine perform during a single cycle?
(a) ***140J
(b) 220J
(c) 360J
(d) 580J
(e) There is not enough information to determine this.
11. If the temperature at which energy is exhauste is Tc = 300K, what is
the temperature at which energy was absorbed?
(a) 117K
(b) 183K
(c) 300K
(d) ***490K
(e) 771K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning