A heat engine takes an ideal monoatomic gas through the cycle in this pV diagram. Step ab is isochoric, bc is isothermal, and ca is isobaric. At point a, the pressure is 2.00 atm, the volume is 2.00 L, and the temperature is 300 K. At point b, the temperature is 600K. a) Calculate the heat added or removed, change in internal energy, and work done for each step and complete the table below.
A heat engine takes an ideal monoatomic gas through the cycle in this pV diagram. Step ab is isochoric, bc is isothermal, and ca is isobaric. At point a, the pressure is 2.00 atm, the volume is 2.00 L, and the temperature is 300 K. At point b, the temperature is 600K. a) Calculate the heat added or removed, change in internal energy, and work done for each step and complete the table below.
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter2: Kinematics
Section: Chapter Questions
Problem 19CQ: What is the last thing you should do when solving a problem? Explain.
Related questions
Question
A
a) Calculate the heat added or removed, change in internal energy, and work done for each step and complete the table below.
b) What is the efficiency of this engine? 1 atm = 1.01×105 Pa
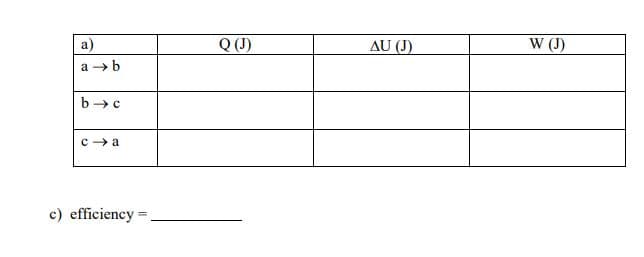
Transcribed Image Text:а)
Q(J)
AU (J)
W (I)
a → b
b →c
с) efficiency -
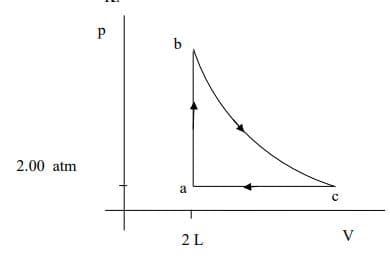
Transcribed Image Text:b
2.00 atm
a
2 L
V
Expert Solution
Step 1
The first law of thermodynamics states that:
| a) | Q(J) | W(J) | |
| a b | 22597.82 | 22597.82 | 0 |
| b c | 9072 | 0 | 9072 |
| c a | -23001.87 | -22597.82 | -404 |
At point a, P= 2 atm = 2.02 X 105 Pascal, V= 2L and T= 300 K
Using the gas equation
For a monoatomic gas
Pressure at b = [ab process]
Volume at c=
Work Done in a Isothermal process =
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University