One mole of an ideal gas passes through the cycle in the figure, which involves a total amount of heat of 440 J. The lowest pressure reached by the gas in the cycle is 100 Pa, and when that happens its temperature is 30 °C. Determine whether the gas absorbs or loses heat in the cycle and the temperature of the gas when its volume is at its maximum. P (Pa) V (m³)
One mole of an ideal gas passes through the cycle in the figure, which involves a total amount of heat of 440 J. The lowest pressure reached by the gas in the cycle is 100 Pa, and when that happens its temperature is 30 °C. Determine whether the gas absorbs or loses heat in the cycle and the temperature of the gas when its volume is at its maximum. P (Pa) V (m³)
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter17: Energy In Thermal Processes: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 36P
Related questions
Question
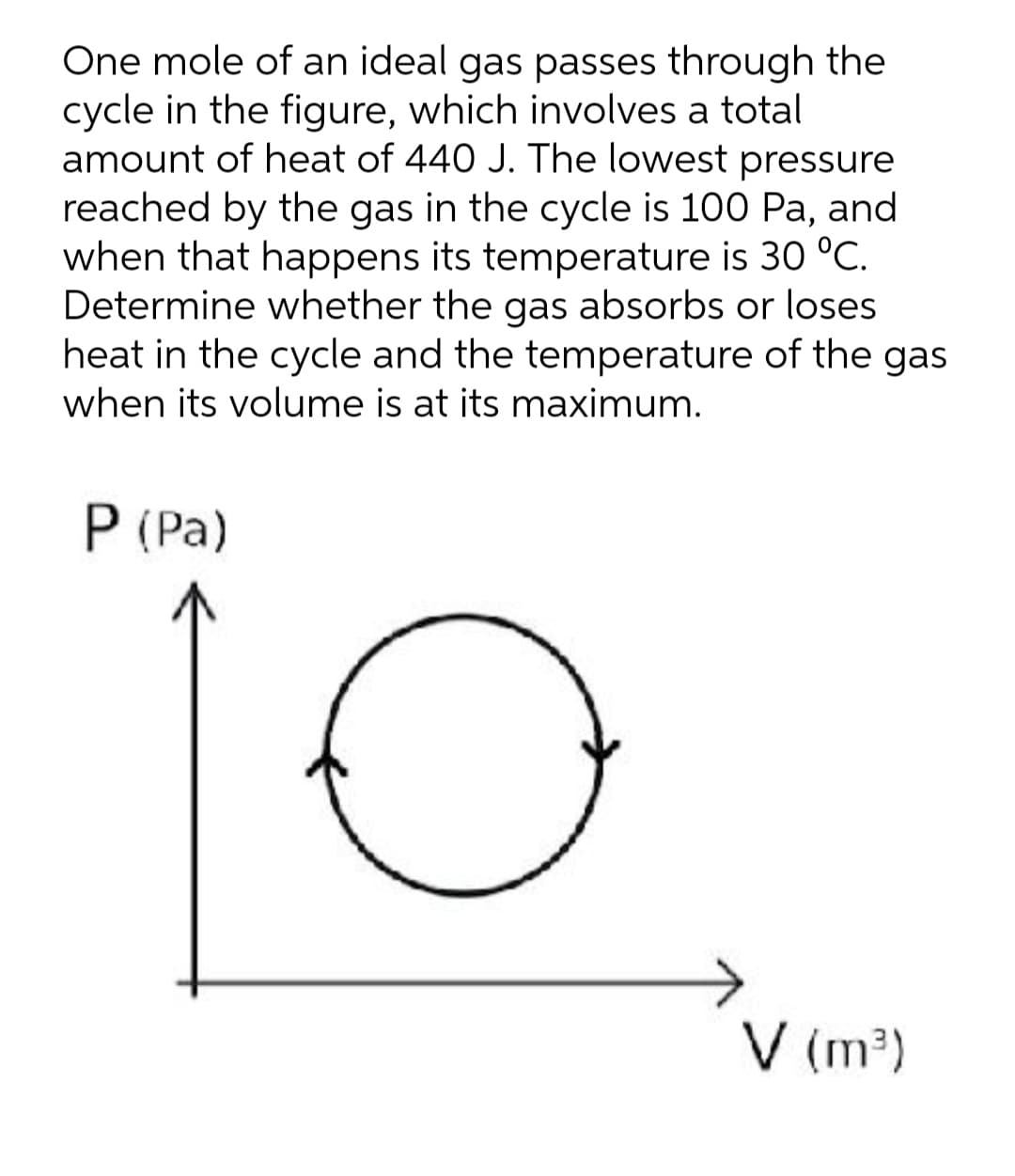
Transcribed Image Text:One mole of an ideal gas passes through the
cycle in the figure, which involves a total
amount of heat of 440 J. The lowest pressure
reached by the gas in the cycle is 100 Pa, and
when that happens its temperature is 30 °C.
Determine whether the gas absorbs or loses
heat in the cycle and the temperature of the gas
when its volume is at its maximum.
P (Pa)
V (m³)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning