A major component of gasoline is octane (C,H18). When liquid octane is burned in air it reacts with oxygen (0,) gas to produce carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce 0.090 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. Dlo
A major component of gasoline is octane (C,H18). When liquid octane is burned in air it reacts with oxygen (0,) gas to produce carbon dioxide gas and water vapor. Calculate the moles of oxygen needed to produce 0.090 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. Dlo
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter1: Essential Ideas
Section: Chapter Questions
Problem 30E: A 2.0-mer volume of hydrogen gas combined with 1.0 liter of oxygen gas to produce 2.0 liters of...
Related questions
Question
100%
please answer quickly thank u
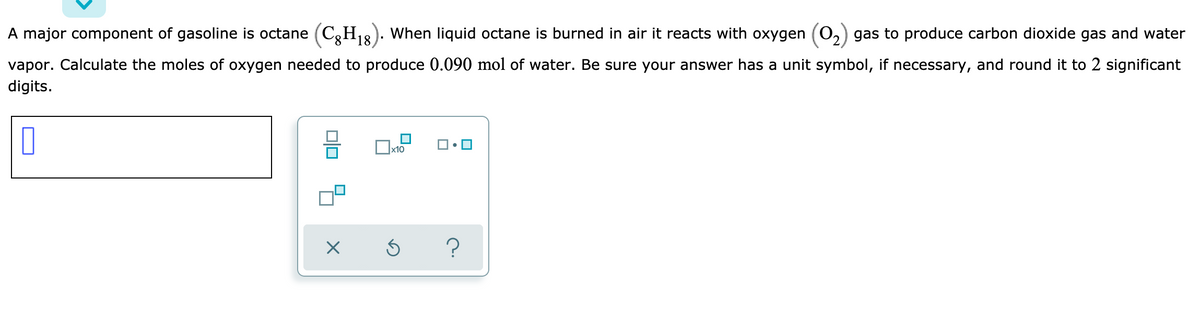
Transcribed Image Text:A major component of gasoline is octane (C,H1&). When liquid octane is burned in air it reacts with oxygen (0,) gas to produce carbon dioxide gas and water
vapor. Calculate the moles of oxygen needed to produce 0.090 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant
digits.
x10
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning