A mechanism for the reaction of bromine with 4,4-dimethylcyclopentene in water is shown below. Which of the following statements about this mechanism is correct? Step 1 Br Br Br + Br Br Step 2 Br OH2 OH2 Br Br OH2 Step 3 + Hо H OH O In Step 1, bromine could add to the other face of the alkene, giving a bromonium ion that is the enantiomer of the one shown. O In Step 2, water could attack the other carbon atom of the bromonium ion, leading to the enantiomer of the product shown. O This mechanism is complete and correct. O In Step 2, water could attack the bromonium ion from the other side, leading to the cis product.
A mechanism for the reaction of bromine with 4,4-dimethylcyclopentene in water is shown below. Which of the following statements about this mechanism is correct? Step 1 Br Br Br + Br Br Step 2 Br OH2 OH2 Br Br OH2 Step 3 + Hо H OH O In Step 1, bromine could add to the other face of the alkene, giving a bromonium ion that is the enantiomer of the one shown. O In Step 2, water could attack the other carbon atom of the bromonium ion, leading to the enantiomer of the product shown. O This mechanism is complete and correct. O In Step 2, water could attack the bromonium ion from the other side, leading to the cis product.
Chapter10: Organohalides
Section10.SE: Something Extra
Problem 20MP: In light of the fact that tertiary alkyl halides undergo spontaneous dissociation to yield a...
Related questions
Question
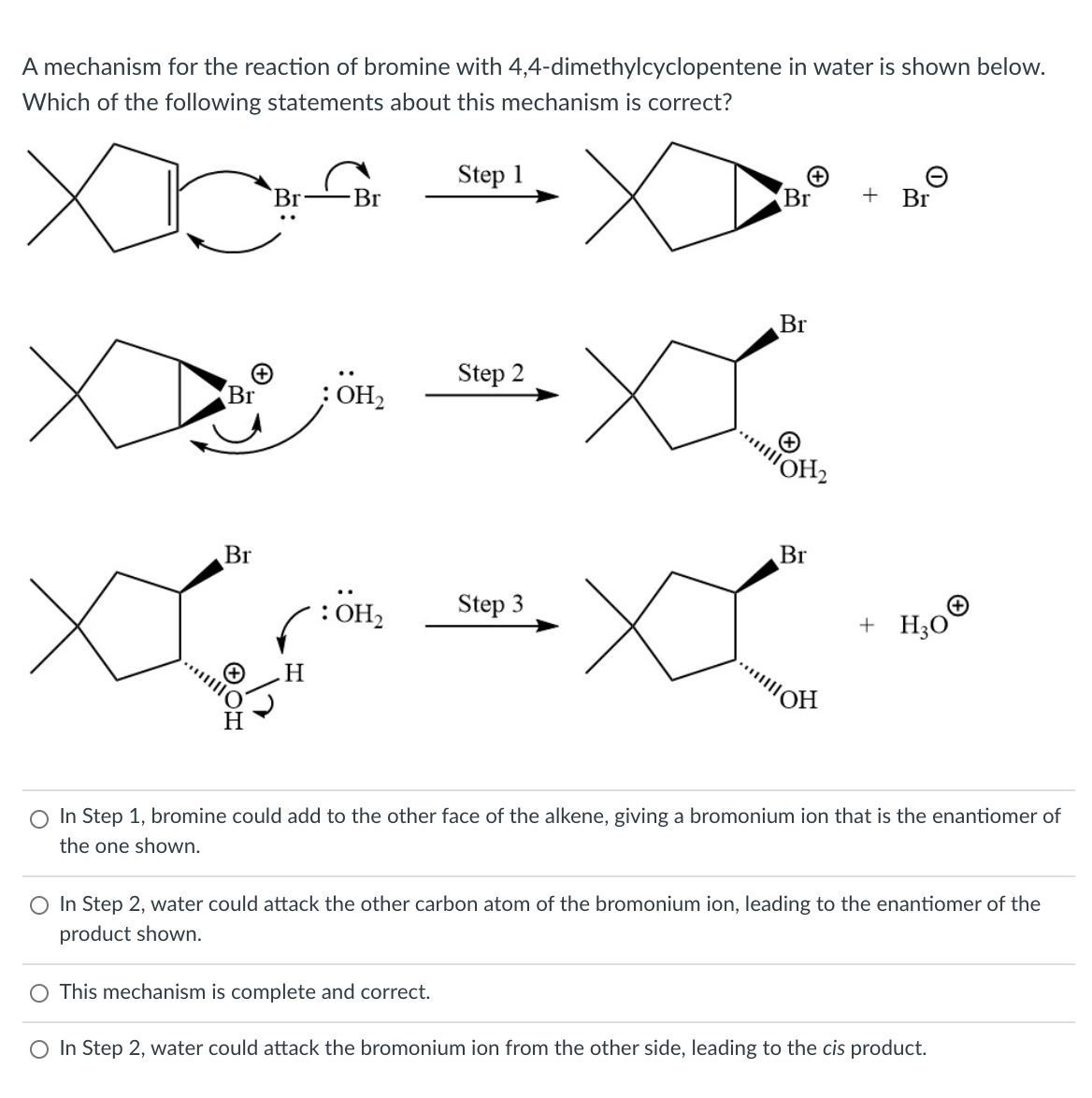
Transcribed Image Text:A mechanism for the reaction of bromine with 4,4-dimethylcyclopentene in water is shown below.
Which of the following statements about this mechanism is correct?
Step 1
Br
Br
Br
+ Br
Br
Step 2
Br
;OH
OH2
Br
Br
XX
..
Step 3
+ Hо
HO:)
H.
O In Step 1, bromine could add to the other face of the alkene, giving a bromonium ion that is the enantiomer of
the one shown.
O In Step 2, water could attack the other carbon atom of the bromonium ion, leading to the enantiomer of the
product shown.
O This mechanism is complete and correct.
O In Step 2, water could attack the bromonium ion from the other side, leading to the cis product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

