Consider mixing 2 of the three compounds to form a solution. i. Which of the compounds, A – C, is most likely to dissolve both of the other compounds? (ie, which compound would be miscible with each of the other compounds) Explain your answer using intermolecular forces. ii. Evaluate the two solutions that the compound in (i) can form with the other compounds. List the solute and solvent of each of the two solutions, then for each solution: 1. Predict if the formation of each of the solutions will be endothermic, exothermic, or ideal. Explain your answer 2. Predict if the vapor pressure of each solution will be greater then, less then, or the same as the vapor pressure predicted by Raoult's law. Explain your answer. (note: all three compounds are volatile) For problem c.ii. you do not need to perform a calculation, but explain each prediction with scientific reasoning and an evaluation of intermolecular forces.
Consider mixing 2 of the three compounds to form a solution. i. Which of the compounds, A – C, is most likely to dissolve both of the other compounds? (ie, which compound would be miscible with each of the other compounds) Explain your answer using intermolecular forces. ii. Evaluate the two solutions that the compound in (i) can form with the other compounds. List the solute and solvent of each of the two solutions, then for each solution: 1. Predict if the formation of each of the solutions will be endothermic, exothermic, or ideal. Explain your answer 2. Predict if the vapor pressure of each solution will be greater then, less then, or the same as the vapor pressure predicted by Raoult's law. Explain your answer. (note: all three compounds are volatile) For problem c.ii. you do not need to perform a calculation, but explain each prediction with scientific reasoning and an evaluation of intermolecular forces.
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter15: Carboxylic Acids And Esters
Section: Chapter Questions
Problem 15.74E
Related questions
Question

Transcribed Image Text:c. Consider mixing 2 of the three compounds to form a solution.
i. Which of the compounds, A - C, is most likely to dissolve both of the other compounds?
(ie, which compound would be miscible with each of the other compounds) Explain your
answer using intermolecular forces.
ii. Evaluate the two solutions that the compound in (i) can form with the other compounds.
List the solute and solvent of each of the two solutions, then for each solution:
1. Predict if the formation of each of the solutions will be endothermic, exothermic,
or ideal. Explain your answer
2. Predict if the vapor pressure of each solution will be greater then, less then, or
the same as the vapor pressure predicted by Raoult's law. Explain your answer.
(not
all three com
unds are
ile)
For problem c.ii. you do not need to perform a calculation, but explain each prediction
with scientific reasoning and an evaluation of intermolecular forces.
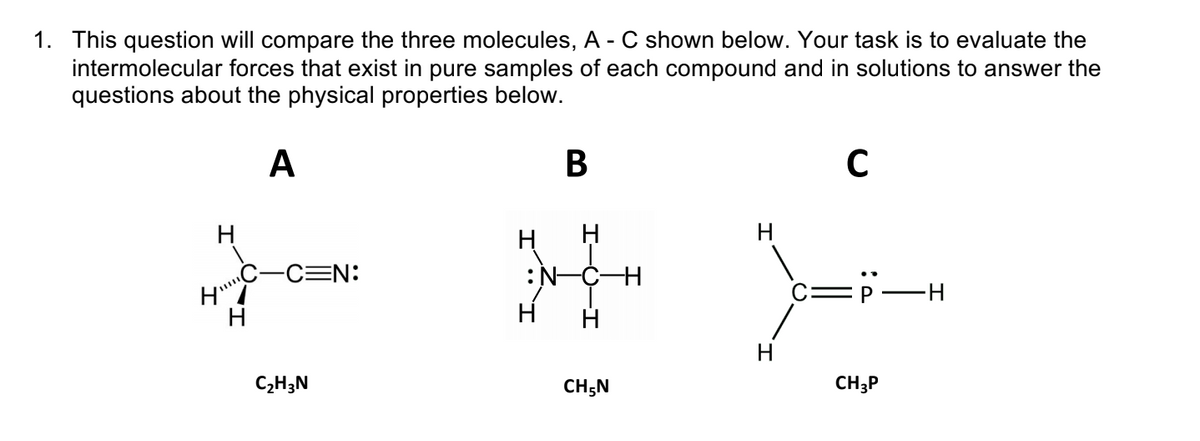
Transcribed Image Text:1. This question will compare the three molecules, A - C shown below. Your task is to evaluate the
intermolecular forces that exist in pure samples of each compound and in solutions to answer the
questions about the physical properties below.
A
В
H
H
CEN:
:N-CH
C=P
H
H
C2H3N
CH;N
CH3P
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning