A nutritional calorie (kcal) is equal to 4.184 kJ. A can of Coca Cola contains 39 g of sugars. We will assume those sugars ultimately all get converted to 39 g of glucose (C6H12O6) in the body. Your body uses glucose in cellular respiration according to the following equation: C6H12O6(s) + 602(g) → 6CO2(g) + 6H₂O (1) a. Using enthalpies of formation provided on the reference page, how many kcal are contained in a can of Coca Cola? You can assume that all the calories in the drink are coming from sugar. b. While carbohydrates (like glucose) and protein provide about 4 nutritional calories per gram consumed, fats (lipids) provide about 9 nutritional calories per gram consumed. What does this tell you about the enthalpy of the reactions that consume fats compared to the enthalpy you determined above for the consumption of glucose?
A nutritional calorie (kcal) is equal to 4.184 kJ. A can of Coca Cola contains 39 g of sugars. We will assume those sugars ultimately all get converted to 39 g of glucose (C6H12O6) in the body. Your body uses glucose in cellular respiration according to the following equation: C6H12O6(s) + 602(g) → 6CO2(g) + 6H₂O (1) a. Using enthalpies of formation provided on the reference page, how many kcal are contained in a can of Coca Cola? You can assume that all the calories in the drink are coming from sugar. b. While carbohydrates (like glucose) and protein provide about 4 nutritional calories per gram consumed, fats (lipids) provide about 9 nutritional calories per gram consumed. What does this tell you about the enthalpy of the reactions that consume fats compared to the enthalpy you determined above for the consumption of glucose?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 112AE: In a bomb calorimeter, the reaction vessel is surrounded by water that must be added for each...
Related questions
Question
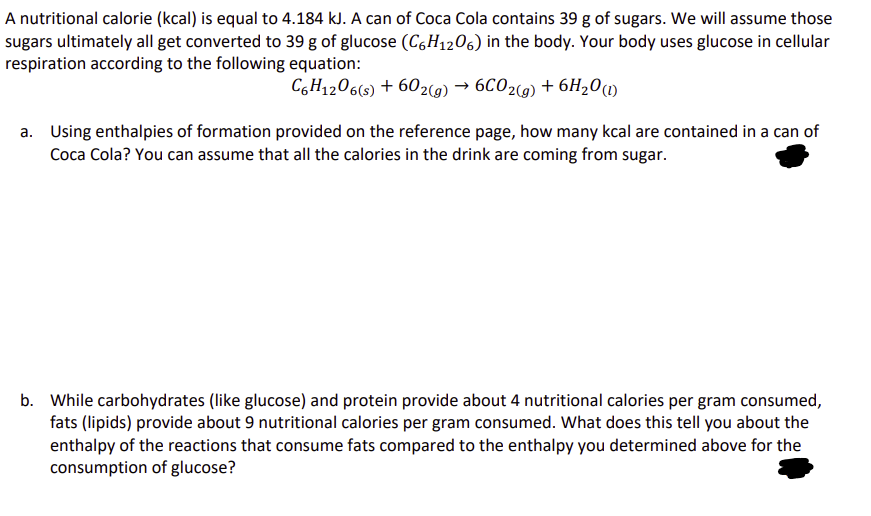
Transcribed Image Text:A nutritional calorie (kcal) is equal to 4.184 kJ. A can of Coca Cola contains 39 g of sugars. We will assume those
sugars ultimately all get converted to 39 g of glucose (C6H12O6) in the body. Your body uses glucose in cellular
respiration according to the following equation:
C6H12O6(s) + 602(g) → 6CO2(g) + 6H₂O (1)
a. Using enthalpies of formation provided on the reference page, how many kcal are contained in a can of
Coca Cola? You can assume that all the calories in the drink are coming from sugar.
b. While carbohydrates (like glucose) and protein provide about 4 nutritional calories per gram consumed,
fats (lipids) provide about 9 nutritional calories per gram consumed. What does this tell you about the
enthalpy of the reactions that consume fats compared to the enthalpy you determined above for the
consumption of glucose?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning