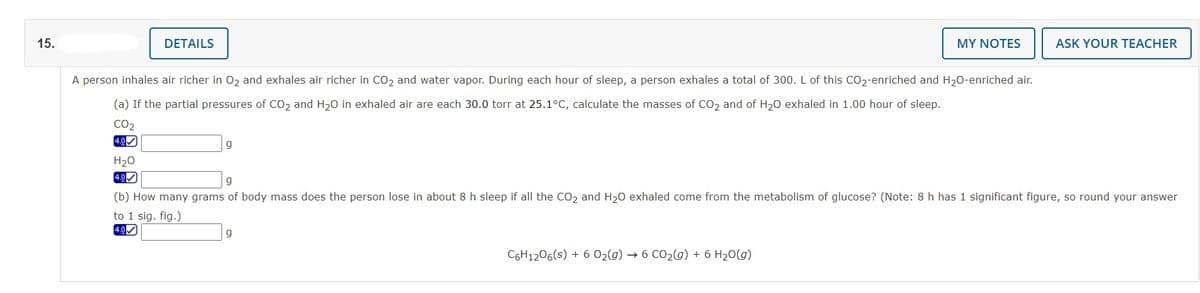
A person inhales air richer in Oz and exhales air richer in CO2 and water vapor. During each hour of sleep, a person exhales a total of 300. L of this CO2-enriched and H20-enriched air. (a) If the partial pressures of Co2 and H20 in exhaled air are each 30.0 torr at 25.1°C, calculate the masses of CO2 and of H20 exhaled in 1.00 hour of sleep. CO2 H20 (b) How many grams of body mass does the person lose in about 8 h sleep if all the CO2 and H20 exhaled come from the metabolism of glucose? (Note: 8 h has 1 significant figure, so round your answer to 1 sig. fig.) CeH1206(s) + 6 02(9) + 6 CO2(g) + 6 H20(g)
A person inhales air richer in Oz and exhales air richer in CO2 and water vapor. During each hour of sleep, a person exhales a total of 300. L of this CO2-enriched and H20-enriched air. (a) If the partial pressures of Co2 and H20 in exhaled air are each 30.0 torr at 25.1°C, calculate the masses of CO2 and of H20 exhaled in 1.00 hour of sleep. CO2 H20 (b) How many grams of body mass does the person lose in about 8 h sleep if all the CO2 and H20 exhaled come from the metabolism of glucose? (Note: 8 h has 1 significant figure, so round your answer to 1 sig. fig.) CeH1206(s) + 6 02(9) + 6 CO2(g) + 6 H20(g)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 94E: Xenon and fluorine will react to form binary compounds when a mixture of these two gases is heated...
Related questions
Question
Transcribed Image Text:15.
DETAILS
MY NOTES
ASK YOUR TEACHER
A person inhales air richer in 02 and exhales air richer in CO2 and water vapor. During each hour of sleep, a person exhales a total of 300. L of this CO2-enriched and H20-enriched air.
(a) If the partial pressures of CO2 and H20 in exhaled air are each 30.0 torr at 25.1°C, calculate the masses of CO2 and of H20 exhaled in 1.00 hour of sleep.
CO2
4.0)
H20
4.0
(b) How many grams of body mass does the person lose in about 8 h sleep if all the Co, and H20 exhaled come from the metabolism of glucose? (Note: 8 h has 1 significant figure, so round your answer
to 1 sig. fig.)
4.0
C6H1206(s) + 6 02(g) → 6 CO2(g) + 6 H20(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning