A: Read each statement below carefully. Place a T on the line if you think a statement is TRUE. Place an F on the line if you think the statement is FALSE. _1. Molecular formulas describe the exact number and type of atoms in a single molecule of a compound. _2. A molecular formula consists of the chemical symbols for the constituent elements followed by numeric subscripts describing the number of atoms of each element present in the molecule. 3. The molecular formula for a compound can be the same as or a multiple of the compound's empirical formula.
A: Read each statement below carefully. Place a T on the line if you think a statement is TRUE. Place an F on the line if you think the statement is FALSE. _1. Molecular formulas describe the exact number and type of atoms in a single molecule of a compound. _2. A molecular formula consists of the chemical symbols for the constituent elements followed by numeric subscripts describing the number of atoms of each element present in the molecule. 3. The molecular formula for a compound can be the same as or a multiple of the compound's empirical formula.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 129GQ: Empirical and molecular formulas. (a) Fluorocarbonyl hypofluorite is composed of 14.6% C, 30.0% O,...
Related questions
Question
??

Transcribed Image Text:Jumpstart
Pre-Test Activity No.1:
A: Read each statement below carefully. Place a T on the line if you think a statement is
TRUE. Place an F on the line if you think the statement is FALSE.
1. Molecular formulas describe the exact number and type of atoms in a single
molecule of a compound.
2. A molecular formula consists of the chemical symbols for the constituent
elements followed by numeric subscripts describing the number of atoms of
each element present in the molecule.
3. The molecular formula for a compound can be the same as or a multiple of
the compound's empirical formula.
4. The molecular formula for glucose is C6H1206.
5. Molecular formulas contain information about the arrangement of atoms. B:
Read each item carefully. Encircle the letter of the correct answer.
1. Which formula shows the actual number of and kinds of atom in one
molecule of a compound?
A. Chemical formula
C. empirical formula
B. Covalent formula
D. molecular formula
2. What is the first step in calculating the molecular formula of a compound?
A. Calculate the molar mass
B. Find the empirical formula
C. Divide the given molar mass by the empirical molar mass
D. Multiply the subscript of the symbols in the empirical formula
3. Which of the following shows molecular formula?
A. NazSO4
B. C3H3
C. N204
D. Als(SO.)2
4. Which is the empirical formula of a substance with molecular formula
C8H10N4O2?
A. CAHSN20
B. CHN20
C. CAHSNO
D. CH:N20
25
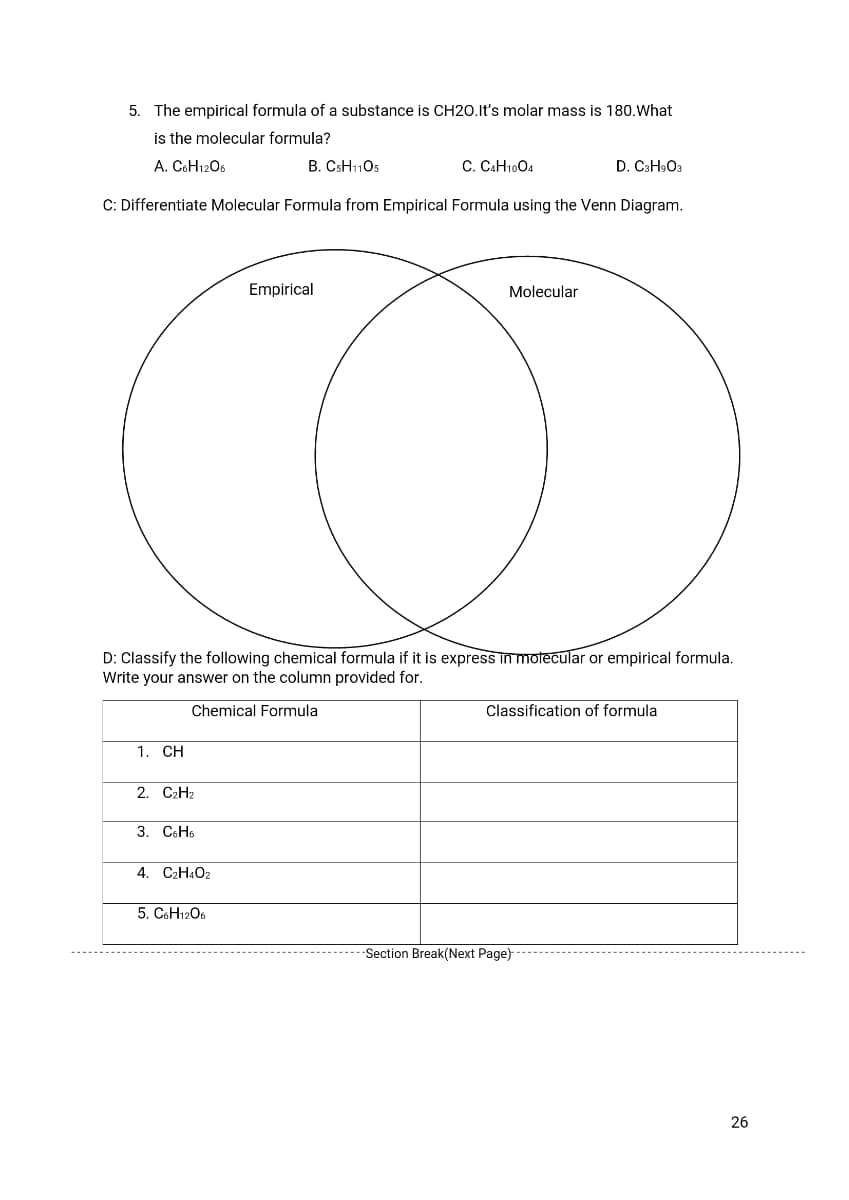
Transcribed Image Text:5. The empirical formula of a substance is CH20.It's molar mass is 180.What
is the molecular formula?
A. C6H1206
B. CSH11O5
C. CAH1004
D. C3H9O3
C: Differentiate Molecular Formula from Empirical Formula using the Venn Diagram.
Empirical
Molecular
D: Classify the following chemical formula if it is express in motecular or empirical formula.
Write your answer on the column provided for.
Chemical Formula
Classification of formula
1. CH
2. C2H2
3. CоНь
4. C2H4O2
5. COH1206
-Section Break(Next Page)
26
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning