A sample of a radioactive substance has an initial mass of 563.6 mg. This substance follows a continuous exponential decay model and has a haif-life of 7 days. (a) Let r be the time (in days) since the start of the experiment, and let y be the amount of the substance at time t. - Olno Write a formula relating y to r. Use exact expressions to fil in the missing parts of the formula. Do not use approximations. (b) How much will be present in 19 days? Do not round any intermediate computations, and round your answer to the nearest tenth.
A sample of a radioactive substance has an initial mass of 563.6 mg. This substance follows a continuous exponential decay model and has a haif-life of 7 days. (a) Let r be the time (in days) since the start of the experiment, and let y be the amount of the substance at time t. - Olno Write a formula relating y to r. Use exact expressions to fil in the missing parts of the formula. Do not use approximations. (b) How much will be present in 19 days? Do not round any intermediate computations, and round your answer to the nearest tenth.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter20: Nuclear Chemistry
Section: Chapter Questions
Problem 65E
Related questions
Question
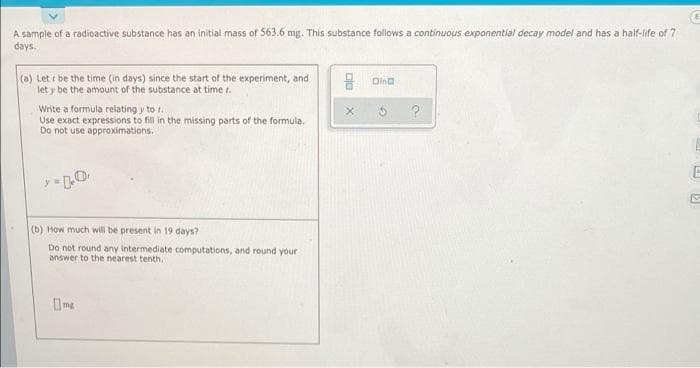
Transcribed Image Text:A sample of a radioactive substance has an initial mass of 563.6 mg. This substance follows a continuous exponential decay model and has a haif-life of 7
days.
(a) Let r be the time (in days) since the start of the experiment, and
let y be the amount of the substance at time r.
Oina
Write a formula relating y to r.
Use exact expressions to fill in the missing parts of the formula,
Do not use approximations.
(b) How much will be present in 19 days?
Do not round any intermediate computations, and round your
answer to the nearest tenth.
O mg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co