A sketch of a phase diagram is given below. Liquid Solid Gas Tempenture Which statement about this diagram is true? a. Increasing temperature at constant pressure below the pressure of Point A can transform the vapor (gas) into solid. b. For the liquid of this substance, increasing pressure at constant temperature can transform liquid into solid . Increasing temperature at constant pressure below the pressure of Point A can transform the liquid into vapor C. (gas). d. For the liquid of this subsance, increasing pressure at constant temperature can transform liquid into vapor (gas). amssH
A sketch of a phase diagram is given below. Liquid Solid Gas Tempenture Which statement about this diagram is true? a. Increasing temperature at constant pressure below the pressure of Point A can transform the vapor (gas) into solid. b. For the liquid of this substance, increasing pressure at constant temperature can transform liquid into solid . Increasing temperature at constant pressure below the pressure of Point A can transform the liquid into vapor C. (gas). d. For the liquid of this subsance, increasing pressure at constant temperature can transform liquid into vapor (gas). amssH
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 117QRT: The phase diagram for water over a relative narrow pressure and temperature range is given in Figure...
Related questions
Question
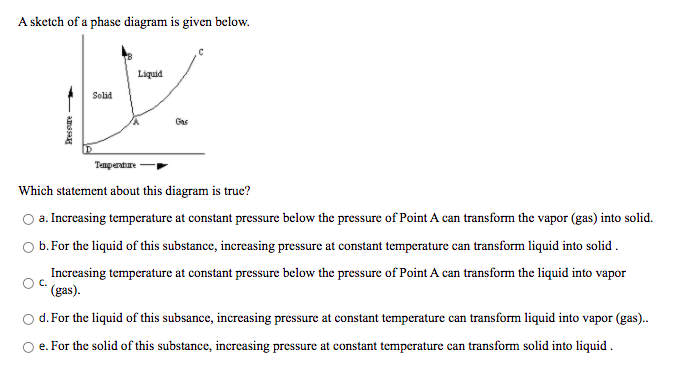
Transcribed Image Text:A sketch of a phase diagram is given below.
Liquid
Solid
Gas
Tempenture
Which statement about this diagram is true?
a. Increasing temperature at constant pressure below the pressure of Point A can transform the vapor (gas) into solid.
b. For the liquid of this substance, increasing pressure at constant temperature can transform liquid into solid .
Increasing temperature at constant pressure below the pressure of Point A can transform the liquid into vapor
(gas).
d.For the liquid of this subsance, increasing pressure at constant temperature can transform liquid into vapor (gas).
O e. For the solid of this substance, increasing pressure at constant temperature can transform solid into liquid .
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning