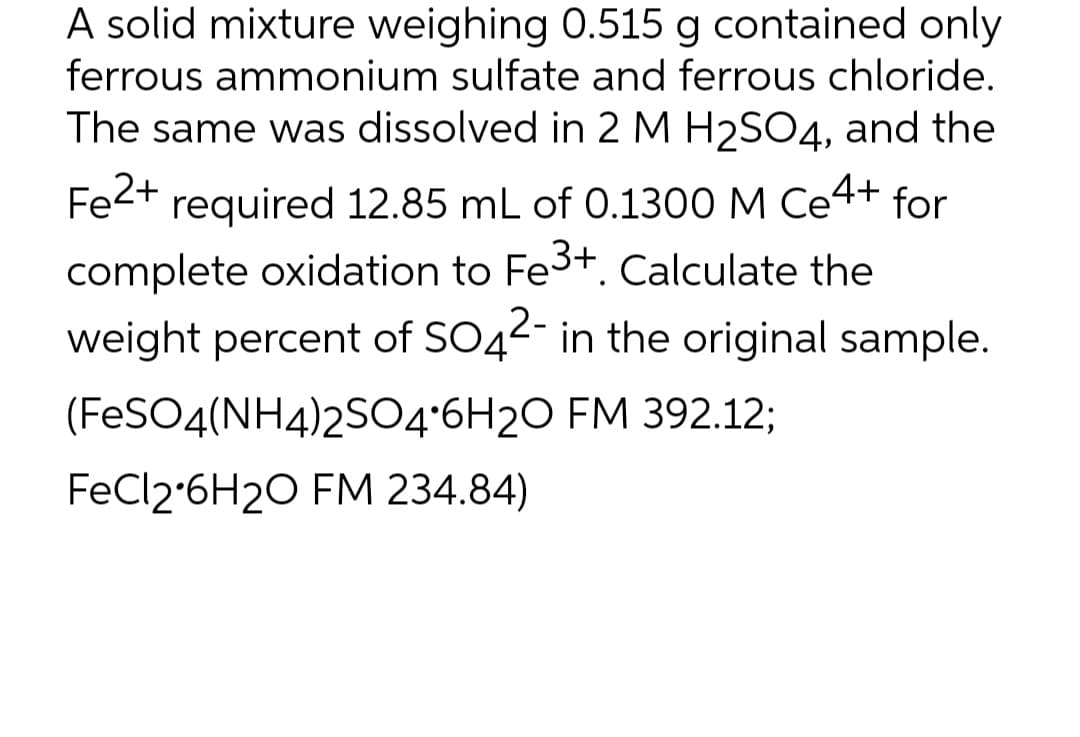
A solid mixture weighing 0.515 g contained only ferrous ammonium sulfate and ferrous chloride. The same was dissolved in 2 M H2SO4, and the Fe2+ required 12.85 mL of 0.1300 M Ce4+ complete oxidation to Fe3+. Calculate the for weight percent of SO42- in the original sample. (FESO4(NH4)2S04'6H2O FM 392.12; FeCl2-6H2O FM 234.84)
Q: Examine the impact of the following circumstance on the PURITY of the sample. Decide whether the…
A: EDTA is ethylenediaminetetraacetic acid which is an indicator. It has 2 amino and 4 carboxyl groups.…
Q: arefully weighed 280mg Calcium carbonate was used in the standardization of an EDTA solution.…
A: Millimoles of a component is calculated by - concentration (mol/L or M) × volume used (mL). While…
Q: 2.054 g of macrogol monostearate (average molecular weight 706. 5) was added to a 200 ml flask and…
A: Solution: We know the saponification reaction i.e the base catalysed hydrolysis reaction of an ester…
Q: Consider a soil with negligible ionic strength (activity = concentration), constant pH of 7.7,…
A:
Q: One litre of a saturated aqueous solution of Ag2SO4 (MW = 311.79 g mol- 1) at 25 °C is evaporated to…
A:
Q: A 0.3045 g of CaCO3 primary standard was dissolved using concentrated HCl, evaporated to incipient…
A: Solution -
Q: The following question is with reference to the following reaction: Fe3+ + SCN minus- [F e(SCN)]2+…
A:
Q: A 0.1093-g sample of impure Na2CO3 was analyzed by the Volhard method. After adding 50.00 mL of…
A:
Q: Drye quantity of primary standard potassim hydrogen phthalate (CHP) for about 2 heat 110°C (Fig.…
A: Given: mass of KHP = 0.7624 g molar mass of KHP = 204.22 g/mol Titration Initial volume (mL)…
Q: A 0.3045 g of CaCO3 primary standard was dissolved using concentrated HCl, evaporated to incipient…
A:
Q: Q1. Dissolved 0.273 grams of pure sodium oxalate (Na:C.O,) in distilled water and added sulfuric…
A: The question is based on the concept of titrations. We are titrating Sodium oxalate with potassium…
Q: The amount of iron in a meteorite was determined by a redox titration using KMnO4 as the titrant. A…
A: Redox titration involves the determination of concentration of a given analyte by using the concept…
Q: You are given a 200-mL sample of surface water from Lake Ontario, and asked to determine the…
A: Given, Volume of water sample = 200mL Volume of K2Cr2O7 = 0.2M Volume of K2Cr2O7 = 15.0 mL Time of…
Q: Q2- A 30.0 mL H2C2O4 acidified solution, was treated with 25.0 mL of 0.102 M KMNO4 solution. The…
A:
Q: Determining Chemical Oxygen Demand You are given a 200-mL sample of surface water from Lake Ontario,…
A: Given, Volume of water sample = 200mL Volume of K2Cr2O7 = 0.2M Volume of K2Cr2O7 = 15.0 mL Time of…
Q: The Tl in a 9.76-g sample of rodenticide was oxidized to the trivalent state and treated with an…
A: a) Direct titration In direct titration , a titrant of known concentration and volume is added to a…
Q: Dissolved 0.273 grams of pure sodium oxalate (NaCO) in distilled water and added sulfuric acid and…
A:
Q: Five white, 500-mg uncoated ascorbic acid (AA) tablets with an average weight of 0.6100-g were…
A: To calculate the amount of ascorbic acid present in a uncoated ascorbic acid tablet having an…
Q: Q3/A 0.80868 gm sample of a commercial phosphate detergent was ignited at a red heat to destroy the…
A: This question is from Analytical Chemistry. In this question we will find out the percentage of P,…
Q: A CaCO3 solution that will be used to standardize EDTA was prepared by dissolving 3.1251 g of solid…
A: Given data; Mass of solid CaCO3 is 3.1251 g The volume of dilute HCl for prepare CaCO3 solution is…
Q: Dissolved 0.273 grams of pure sodium oxalate (Na2C204) in distilled water and added sulfuric acid…
A:
Q: Calculate the gravimetric factor of the following. 2 Fe3O4 is sought(Analyte), 3 Fe2O3 is weighed…
A: Given, Sought : Fe3O4 Precipitate : Fe2O3
Q: QUESTIOΝ1 a) Calculate ionic strength (Im) and mean activity coefficient (Y+) for a 0.050 m solution…
A:
Q: 1093-g sample of impure Na2CO3 was analyzed by residual precipitimetry. After adding 50.00 mL of…
A:
Q: A 500.0mg of butter was warmed and shaken vigorously with water. The undissolved material was…
A: A Volhard method is an inorganic quantitative analytic method in which an indirect or back titration…
Q: Traces of aniline can be determined by reaction with an excess of electrolytically generated Br2:…
A: Given, Current(I) = 1.00 mA= 1.00 x 10-3 A. Generation time for anode = 3.76 min. Generation time…
Q: Ten dietary iron tablets with a total mass of 11.066 g were ground and mixed thoroughly. Then 2.998…
A: Given: 10 dietary tablets Mass of 10 dietary tablets = 11.066 g Now, 2.998 g of powdered tablets…
Q: The weight of 7 ml of vinegar sample was 7.99 g, was required 31.0 ml of 0.3 M sodium hydroxide…
A: Now, the chemical reaction taking place is- CH3COOH + NaOH→CH3COONa + H2O Thus, 1 mole of acetic…
Q: A sample weighing 650 mg containing KCI, BaCl2 and inert materials was dissolved in sufficient water…
A: Given, mass of sample = 650 mg =0.650 g Volume of AgNO3 = 0.1510 M Molarity of AgNO3 = 35.50 mL =…
Q: A 2.067 g of macrogol monostearate (average molecular weight 706. 5) was added to a 200 ml flask and…
A: Solution: We know the macrogol monostearate is the ester of stearic acid. Saponification is the…
Q: A 0.64 g sample containing KCI ( mw = 74.6) is dissolved in 50mL of water and titrated to the…
A: The %W/W of KCl in the solution has to be given,
Q: Orthophosphate (PO4) in river sediment sample is determined by weighing ammonium phosphomolybdate,…
A: Gravimetric factor (G.F.) is the weight of analyte per unit mass of precipitate. It is the ratio of…
Q: A first-stage recovery of magnesium from seawater is precipitationof Mg1OH22 with CaO:Mg2+(aq) +…
A: It is given that Mg(OH)2 is precipitated with the help of CaO(calcium oxide) and the reaction is…
Q: A 46.4046.40 mL aliquot from a 0.4850.485 L solution that contains 0.3500.350 g of MnSO4MnSO4…
A: [(0.450 g MgSO4 ) / (120.37 g MgSO4 /mol)] x [(0.0500 L / 0.5000 L) ÷ (0.0376 L EDTA)] x [(1 mol…
Q: A foot powder sample containing Zn was dissolved on 50.00 ml water and was titrated to the end point…
A:
Q: For titration for determination of Chloride, 0.05 AgNO3 and NaCl with a 5% (w/v) K2CrO4 indicator,…
A: In the determination of chloride ion concentration by Mohr's method, NaCl is used as the primary…
Q: A 0.6025 g sample was dissolved and the Ca2+ and Ba2+ ions present were precipitated as BaC2O4.H2O…
A:
Q: A 0.3045 g of CaCO3 primary standard was dissolved using concentrated HCl, evaporated to incipient…
A: Given, 0.3045 g of CaCO3 dissolved in concentrated HCl Dilute to 250 mL Molar mass of CaCO3 =…
Q: Calculate the AH° of the borate dissolution at 25°C in kJ mol1, if the 22.12 mL of 0.025 M HCI…
A: Solution- Given data- T= 250C =298K ∆S0 = 61.14 J mol-1 K-1, gas constant = 8.314 J mol-1 K-1
Q: The Tl in a 9.76-g sample of rodenticide was oxidized to the trivalent state and treated with an…
A: Tl3+ was titrated with an unmeasured excess of Mg/EDTA solution.
Q: 3. In a preliminary experiment, a solution containing 0.083M X and 0.0666M S gave peak areas of A, =…
A: Given data: For solution: Ax = 423 As = 347 For the mixture: Ax = 553 As = 582
Q: The amount of iron in a meteorite was determined by a redox titration using KMnO4 as the titrant. A…
A:
Q: - A sample of feldspar weighing 1.500g is decomposed, and eventually there is obtained a mixture of…
A: Amount of feldspar = 1.500 g Mixture of NaCl and KCl = 0.1801 g Concentration of AgNO3 =0.08333 N…
Q: The conductivity of a saturated solution of SRSO4 at 298 K is found to be 1.482 x 104 S cm', while…
A: Given data : Conductivity of SrSO4(κ)=1.482×10-4Scm-1λ∞m(Sr2+)=118.92 Scm2mol-1λ∞m(SO42-)=159.6…
Q: The Ksp values of two solid carbonates, A2CO3 and BCO3, were determined by preparing 250.0-mL…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: Q1. Dissolved 0.273 grams of pure sodium oxalate (Na2C0.) In distilled water and added sulfuric acid…
A: The question is based on redox titrations. we have added excess of KMnO4 to the Sodium oxalate. the…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- Six iron tablets containing FeSO4.7H2O were dissolved in 100-ml of 0.1M HNO3 with gentle heating. All of the Fe2+ is converted to Fe3+ by the strong oxidizing conditions. After the solution had cooled to room temperature , 2.5-ml of 35wt% NH4OH was added. The precipitate Fe2O3-xH2O that was filtered weighed 0.345g. Thermogravimetric analysis of the crude product showed a 10.5% weight loss . A. How many waters of hydration were in the precipitate B. How much iron is present in each tabletThe concentration of ammonia in a cleaning product was determined by back titration.Firstly, 10.00 cm3 of the cleaning product was pipetted into a large conical flask,containing 250.00cm3 of 0.50 mol/l HCl to give Solution A.Following a period of reaction and shaking, 50.00cm3 of Solution A was removed anddiluted to 250 cm3 with water in a volumetric flask to give Solution B.20 cm3 samples of Solution B were titrated against 0.05 mol/l Na2CO3 solution, givingan average titre of 12.45 cm3. i) Write equations for the reactions that have taken place.ii) Determine the concentration of NH3 in the original cleaning product in mol/l,g/l, ppm, and % w/v.The amount of iron in a meteorite was determined by a redox titration using KMnO4 as the titrant. A 0.4185 -g sample was dissolved in acid and the liberated Fe3+ quantitatively reduced to Fe2+, using a reductor column. Titrating with 0.0051 M KMnO4 requires 23.44 mL to reach the end point. Determine the %w/w Fe2O3 in the sample of meteorite. (Fe = 55.845 amu, Fe2O3 = 159.69 g/mol
- what wt. of limestone containing 9.57% Mg must be taken for analysis in order to precipitate of 0.551g Mg2P2O7? how many grams of Na2SO4 are required to ppte Ag2SO4 from 2.000t of AgNO3? a sample of magnetite (impure Fe3O4) weighing 0.5000g is fused with oxidizing flux and the ferric compound formed is eventually precipitated as ferric hydroxide and ignited to ferric oxide which weighs 0.4980calculate %Fe & %Fe2O3A sample of an iron ore was prepared for Fe3+ analysis as following: 3.4g of the sample was added anddissolved in acid environment then diluted to 250 mL using volumetric flask. After that, 10 mL of the resultingsolution was transferred by pipet to a 50-mL volumetric flask and continue to be diluted. The scientists foundout that this solution gives the concentration of Fe3+ as 2.3 mg/L. Find the weight percentage of Fe3+ in theoriginal sample.A 0.4020 g sample was dissolved, and Ca2+ and Ba2+ ions present were precipitated as Ba.C2O4.H2O and CaC2O4H2O. The Oxalates were then heated in a thermogravimetric apparatus leaving a residue that weighed 0.3175 g in the range of 320°C to 440°C and 0.2165 g in the range of 580°C to 620°C. Calculate percentage Ca and percentage Ba in the sample.
- An exhausted zeolite softener was regenerated by passing 100 litres, of NaCl. Solution containing 150 gm per lit. of NaCl. How many lit. of a sample of H2O of hardness 300 ppm can be softened by this softener? (Given at wts. for C = 12, O = 16, Na = 23, CI = 35.5, Ca = 40).(i) You have been provided with a sample collected from a water pool beside a slag heap at the iron ore mine. 35 uL of the sample is added to 1165 uL of buffered ferrozine (excess). If the %T recorded at 562 nm in a 2 mm cuvette is found to be 25%, calculate the concentration of Fe(II) in the sample in mol L−1 and ppm. Comment on the contamination level. (ii) Comment on a possible contaminant that could interfere with the assay and suggest an approach that could be used to account for its presence.A 1.000 g sample containing chlorides, iodides and inert materials was treated with dilute nitric acid followed by AgNO3. A precipitate of AgCl (143.32) and AgI (234.77) was produced and weighs 0.9238 g. On heating in a current of Cl2, the AgI is converted to AgCl, and the resulting product weighs 0.7238 g. Find the percentage of a) NaI (149.89) and b) NaCl (58.44) in the sample
- Exactly 6.00 ml of acetic acid (density = 1.05 g/mL), MW =60.05) was added to a 1 –liter volumetric flask, and the flask was filled to the mark with distilled water. A portion of the resulting solution was added to a conductance cell (k = 1.25 cm-1), and the conductance was found to be 416 μS. Calculate the dissociation constant Ka of acetic acid. ΛoH+= 349.8 Scm2/mole and ΛoCH3COO- = 41 Scm2/moleFor Indirect Iodometric Analysis of Copper... ~0.0896g KIO3 necessary to consume 350mL of 0.1 M Na2S2O3, Na2S2O3 is stored in an amber glass bottle until ready for use. Primary Standard KIO3 has 2g of KI, 50mL of DI water, and 10 mL of 1.0M HCl is added then immediately titrated with Na2S2O3 until medium yellow or straw... then 5mL of starch indicator is added and titrated again until blue black color turns clear. Unknown CuO use 1.2G of Unknown, 20mL of HNO3 heated until sample dissolved, 25 mL of DI water added and boiled until clear light blue color, after cooling 1:1 NH3 added (~34.47 mL of NH4OH reagent) until permanent deep blue color amine complex, 2g of NH4HF2 added and swirled until dissolved, 3 g of KI is added then titrated immediately with Na2S2O3 until brown color of iodide is nearly gone (brown milk color), 2 g of KSCN and 3 mL of starch indicator is then added with titration continuing until disappearance of new blue black color. 1. Na2CO3 is often added to thiosulfate…For Indirect Iodometric Analysis of Copper... ~0.0896g KIO3 necessary to consume 350mL of 0.1 M Na2S2O3, Na2S2O3 is stored in an amber glass bottle until ready for use. Primary Standard KIO3 has 2g of KI, 50mL of DI water, and 10 mL of 1.0M HCl is added then immediately titrated with Na2S2O3 until medium yellow or straw... then 5mL of starch indicator is added and titrated again until blue black color turns clear. Unknown CuO use 1.2G of Unknown, 20mL of HNO3 heated until sample dissolved, 25 mL of DI water added and boiled until clear light blue color, after cooling 1:1 NH3 added (~34.47 mL of NH4OH reagent) until permanent deep blue color amine complex, 2g of NH4HF2 added and swirled until dissolved, 3 g of KI is added then titrated immediately with Na2S2O3 until brown color of iodide is nearly gone (brown milk color), 2 g of KSCN and 3 mL of starch indicator is then added with titration continuing until disappearance of new blue black color. 4. Why is the starch indicator solution…

