Drye quantity of primary standard potassim hydrogen phthalate (CHP) for about 2 heat 110°C (Fig. 35-9), and cool in a desiccator. Weigh individual 0,7 to 0.8 g somples (to the nearest 0.Img) into 250 mi conical flasks, and dissolve each in 50 to 75 mL of distilled water. Add 2 drops of phenolphthalein; titrate with base until the pink colour of the indicator persists for 30 seconds (Note). Calculate the concentration of the NaOH solution.
Drye quantity of primary standard potassim hydrogen phthalate (CHP) for about 2 heat 110°C (Fig. 35-9), and cool in a desiccator. Weigh individual 0,7 to 0.8 g somples (to the nearest 0.Img) into 250 mi conical flasks, and dissolve each in 50 to 75 mL of distilled water. Add 2 drops of phenolphthalein; titrate with base until the pink colour of the indicator persists for 30 seconds (Note). Calculate the concentration of the NaOH solution.
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
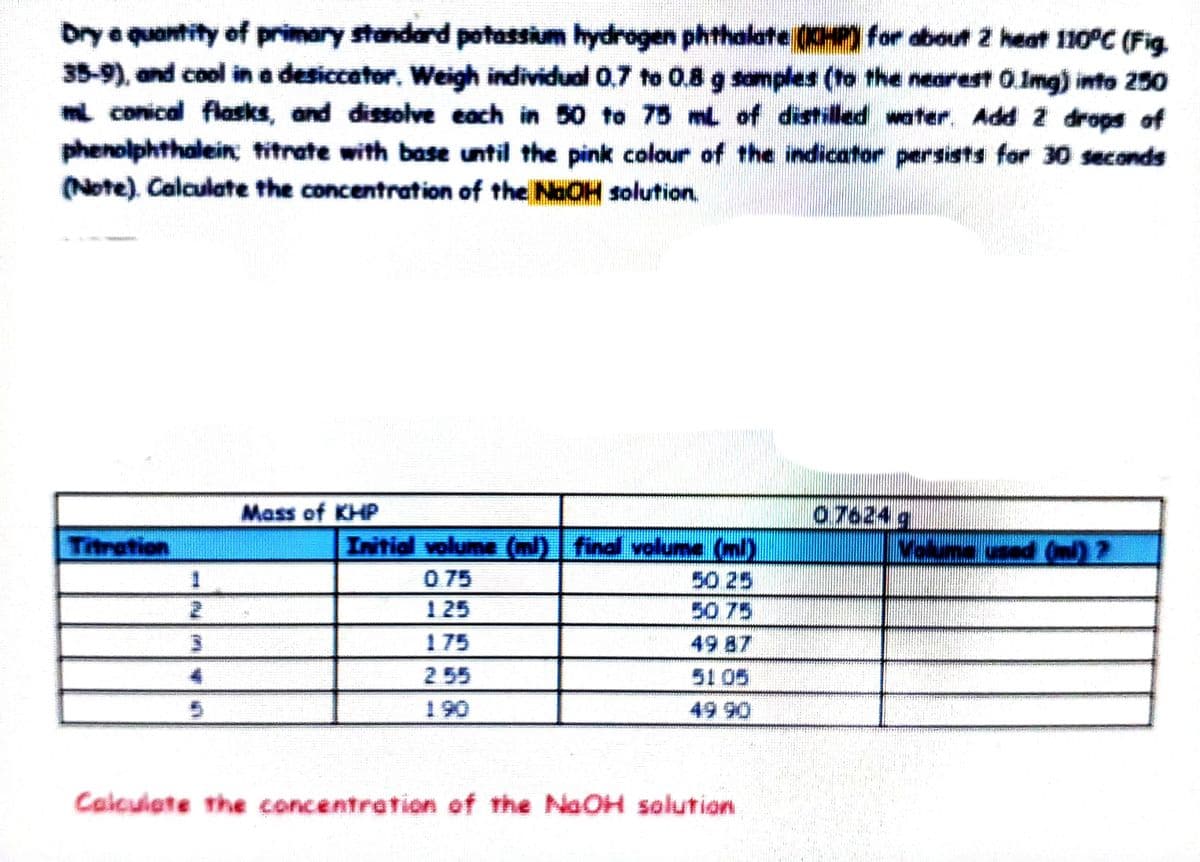
Transcribed Image Text:Drye quantity of primary standard potassium hydrogen phthalate (0HP) for about 2 heat 110°C (Fig
35-9), and cool in a desiccator. Weigh individual 0,7 to 0.8 g samples (to the nearest 0.Img) into 250
i conical flasks, and dissolve each in 50 to 75 mL of distiled water. Add 2 drops of
phenolphthalein; titrate with base until the pink colour of the indicator persists for 30 seconds
(Note). Calculate the concentration of the NaOH solution,
07624 g
Velume ced (n)?
Mass of KHP
Initial volume () find volume (ml)
50 25
50 75
Titration
0 75
125
175
49 87
2 55
51 05
190
49 90
Calculate the concentration of the NaOH solution
Expert Solution
Step by step
Solved in 9 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning