A student uses a strip of metal in an electrolysis circuit in which the metal strip serves as the anode and is connected to the positive terminal of a power supply. The negative terminal of the power supply is connected to an insulated copper wire. An ammeter is placed in the circuit and the power supply is turned on. A current of 1.07 A flows for 5 min and 52 sec. During this time the mass loss of the metal is 0.102 g. What is the molar mass of the metal?
A student uses a strip of metal in an electrolysis circuit in which the metal strip serves as the anode and is connected to the positive terminal of a power supply. The negative terminal of the power supply is connected to an insulated copper wire. An ammeter is placed in the circuit and the power supply is turned on. A current of 1.07 A flows for 5 min and 52 sec. During this time the mass loss of the metal is 0.102 g. What is the molar mass of the metal?
Chapter11: Dynamic Electrochemistry
Section: Chapter Questions
Problem 1P
Related questions
Question
urgent in 30 min
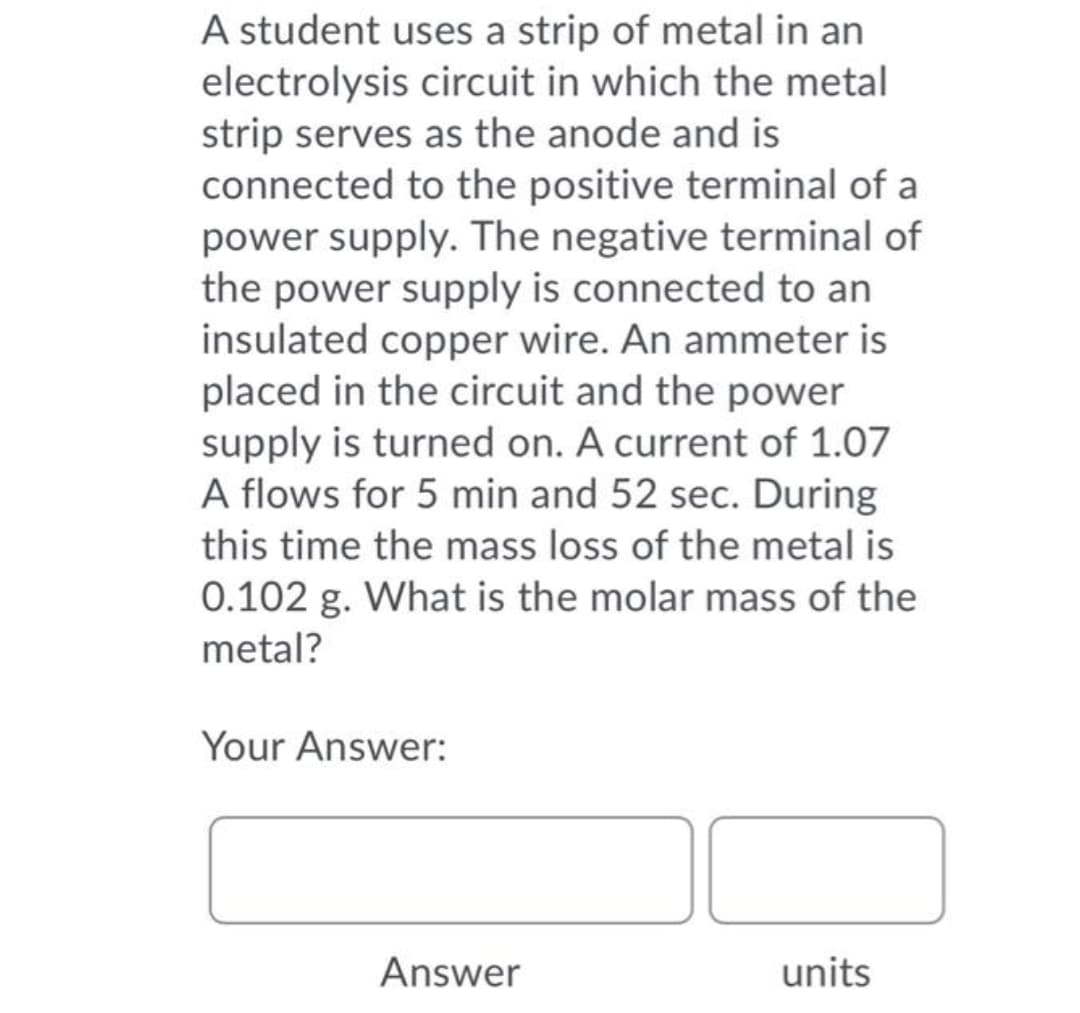
Transcribed Image Text:A student uses a strip of metal in an
electrolysis circuit in which the metal
strip serves as the anode and is
connected to the positive terminal of a
power supply. The negative terminal of
the power supply is connected to an
insulated copper wire. An ammeter is
placed in the circuit and the power
supply is turned on. A current of 1.07
A flows for 5 min and 52 sec. During
this time the mass loss of the metal is
0.102 g. What is the molar mass of the
metal?
Your Answer:
Answer
units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning