A voltaic cell is constructed in which the anode is a Mg|Mg²+ half cell and the cathode is a NiNi2+ half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Use the pull-down boxes to specify states such as (ag) or (s). If a box is not needed, leave it blank.) The anode reaction is: The cathode reaction is: The net cell reaction is: In the external circuit, electrons migrate the NiNi?+ electrode the Mg|Mg?+ electrode. In the salt bridge, anions migrate the Mg|Mg-+ compartment A the NiNi<+ compartment. In the external circuit, electrons migra v the NiNi?+ electrode the Mg|Mg²+ electrode. from In the salt bridge, anions migrate Mg²+ compartment| to a the NilNi2+ compartment.
A voltaic cell is constructed in which the anode is a Mg|Mg²+ half cell and the cathode is a NiNi2+ half cell. The half-cell compartments are connected by a salt bridge. (Use the lowest possible coefficients. Use the pull-down boxes to specify states such as (ag) or (s). If a box is not needed, leave it blank.) The anode reaction is: The cathode reaction is: The net cell reaction is: In the external circuit, electrons migrate the NiNi?+ electrode the Mg|Mg?+ electrode. In the salt bridge, anions migrate the Mg|Mg-+ compartment A the NiNi<+ compartment. In the external circuit, electrons migra v the NiNi?+ electrode the Mg|Mg²+ electrode. from In the salt bridge, anions migrate Mg²+ compartment| to a the NilNi2+ compartment.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
100%
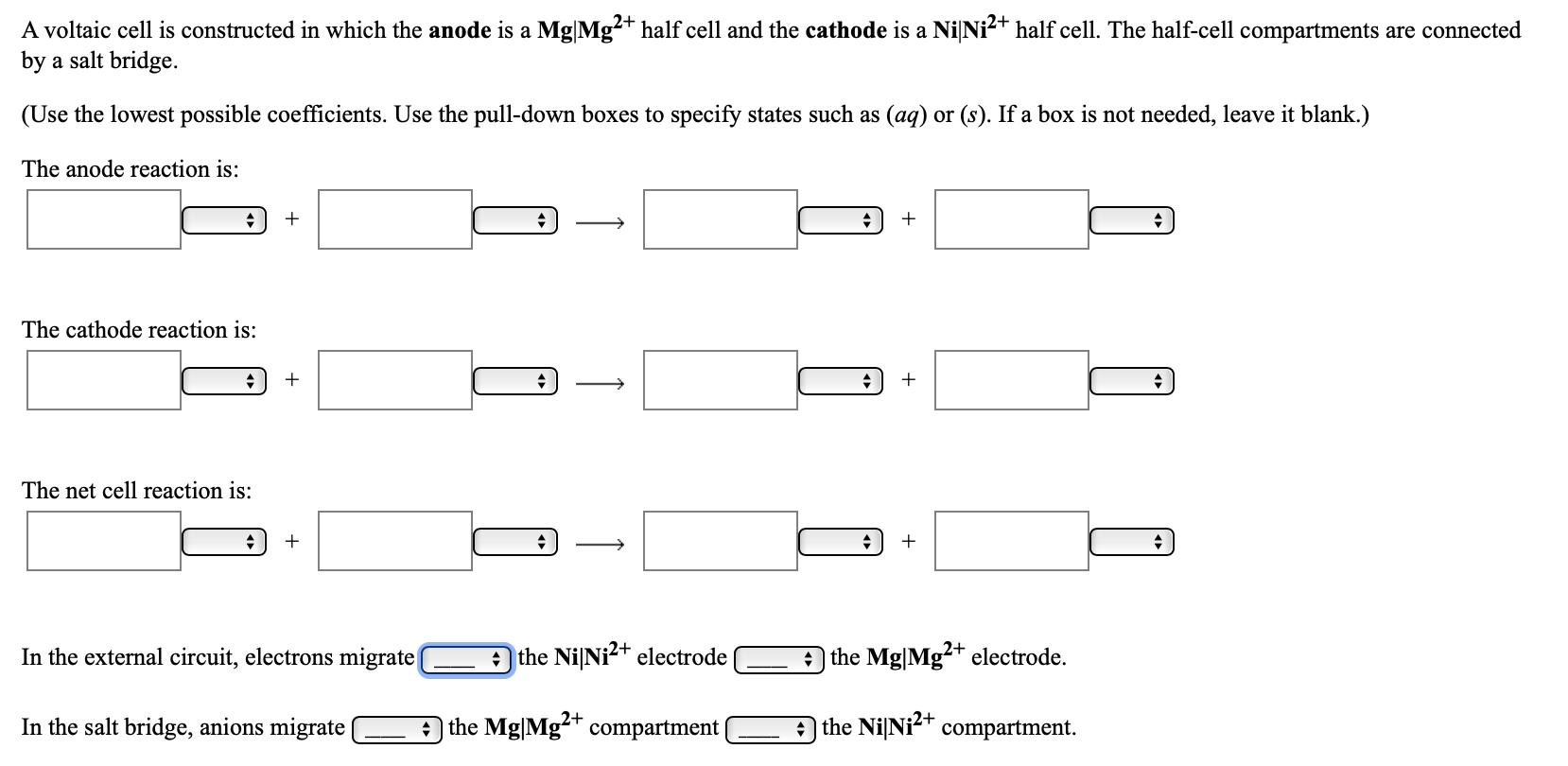
Transcribed Image Text:A voltaic cell is constructed in which the anode is a Mg|Mg²+ half cell and the cathode is a NiNi2+ half cell. The half-cell compartments are connected
by a salt bridge.
(Use the lowest possible coefficients. Use the pull-down boxes to specify states such as (ag) or (s). If a box is not needed, leave it blank.)
The anode reaction is:
The cathode reaction is:
The net cell reaction is:
In the external circuit, electrons migrate
the NiNi?+ electrode
the Mg|Mg?+ electrode.
In the salt bridge, anions migrate
the Mg|Mg-+ compartment
A the NiNi<+ compartment.
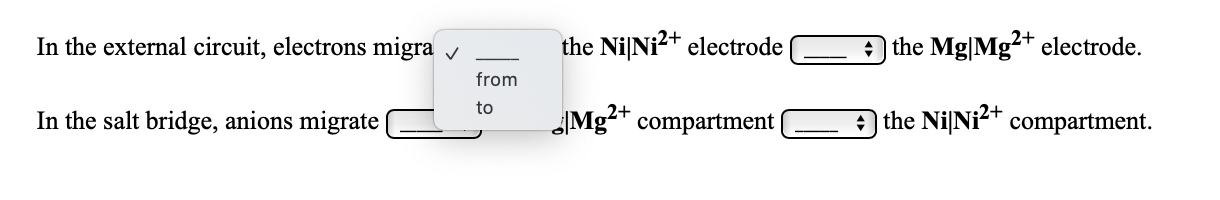
Transcribed Image Text:In the external circuit, electrons migra v
the NiNi?+ electrode
the Mg|Mg²+ electrode.
from
In the salt bridge, anions migrate
Mg²+ compartment|
to
a the NilNi2+ compartment.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning