a. At equilibrium, the product mixture contains about 24% STAR material and about 75% product. Calculate Keq for this reaction. b. What is the approximate value of AGSuperseript double daggero C. Add curved arrows to the mechanism. d. Draw the energy diagram for the reaction.
a. At equilibrium, the product mixture contains about 24% STAR material and about 75% product. Calculate Keq for this reaction. b. What is the approximate value of AGSuperseript double daggero C. Add curved arrows to the mechanism. d. Draw the energy diagram for the reaction.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 18E
Related questions
Question
V4
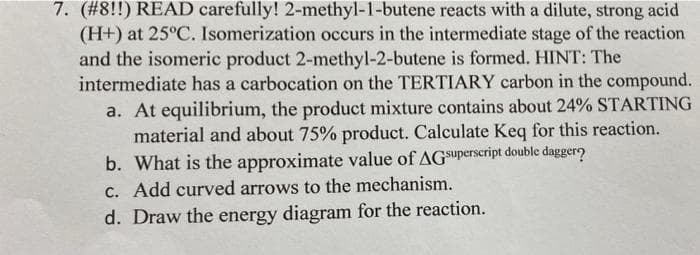
Transcribed Image Text:7. (#8!!) READ carefully! 2-methyl-1-butene reacts with a dilute, strong acid
(H+) at 25°C. Isomerization occurs in the intermediate stage of the reaction
and the isomeric product 2-methyl-2-butene is formed. HINT: The
intermediate has a carbocation on the TERTIARY carbon in the compound.
a. At equilibrium, the product mixture contains about 24% STARTING
material and about 75% product. Calculate Keq for this reaction.
b. What is the approximate value of AGSuperscript double daggero
C. Add curved arrows to the mechanism.
d. Draw the energy diagram for the reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning