a. graph C, vs T then using excel what polynomial trendline is needed to get a fit of R? = 0.999? Using this polynomial what would be the heat of a process heating 3.189 mol of nitrogen gas from 461 K to 917 K? b. Fit the data above using what many texts do: Cy = A + BT +C/T?. This is something likely to be new to you using excel. You will have to have a column for Tand an adjacent column for 1/T?. Then a column for Cy. Go into Data analysis under tools and choose regression. Enter in your Cy column for y and select BOTH the T and 1/T2 columns for your x values. Choose a square in your excel sheet to place the answers. The A, B and C values will be in there! What would the heat of the process from part a be now if using this C(T)?
a. graph C, vs T then using excel what polynomial trendline is needed to get a fit of R? = 0.999? Using this polynomial what would be the heat of a process heating 3.189 mol of nitrogen gas from 461 K to 917 K? b. Fit the data above using what many texts do: Cy = A + BT +C/T?. This is something likely to be new to you using excel. You will have to have a column for Tand an adjacent column for 1/T?. Then a column for Cy. Go into Data analysis under tools and choose regression. Enter in your Cy column for y and select BOTH the T and 1/T2 columns for your x values. Choose a square in your excel sheet to place the answers. The A, B and C values will be in there! What would the heat of the process from part a be now if using this C(T)?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 70AP
Related questions
Question
Please give answer complete questions
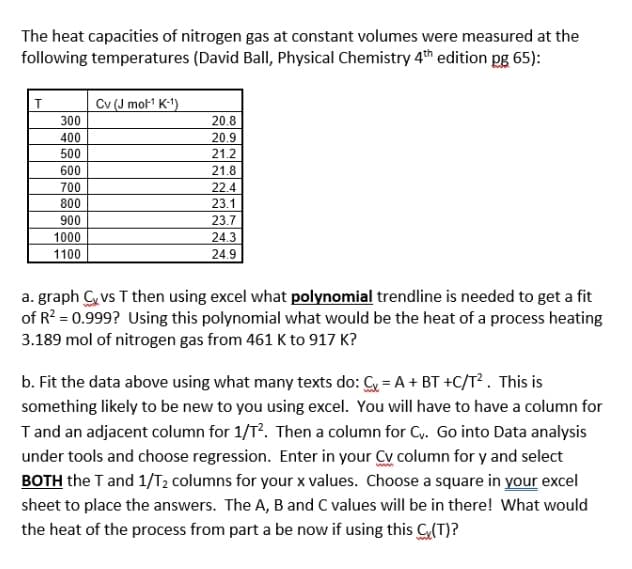
Transcribed Image Text:The heat capacities of nitrogen gas at constant volumes were measured at the
following temperatures (David Ball, Physical Chemistry 4th edition pg 65):
Cv (J mol1 K-1)
300
20.8
400
20.9
21.2
500
600
21.8
700
22.4
800
23.1
900
23.7
1000
24.3
1100
24.9
a. graph C vs T then using excel what polynomial trendline is needed to get a fit
of R? = 0.999? Using this polynomial what would be the heat of a process heating
3.189 mol of nitrogen gas from 461 K to 917 K?
b. Fit the data above using what many texts do: Cy = A + BT +C/T² . This is
something likely to be new to you using excel. You will have to have a column for
Tand an adjacent column for 1/T?. Then a column for Cy. Go into Data analysis
under tools and choose regression. Enter in your Cy column for y and select
BOTH the T and 1/T2 columns for your x values. Choose a square in your excel
sheet to place the answers. The A, B and C values will be in there! What would
the heat of the process from part a be now if using this G(T)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 24 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning