a. Magnesium chloride and sodium hydroxide. OMagnesium hydroxide, Mg(OH)2. Rule 5: Most hydroxide salts are only slightly soluble. OMagnesium hydroxide, Mg(OH)2. Rule 5: Exception. OMagnesium hydroxide, Mg(OH)2. Rule 3: Most hydroxide salts are only slightly soluble. ONo precipitate. b. Copper(II) nitrate and ammonium sulfide. Copper(II) sulfide, CuS. Rule 6: Most sulfide salts are only slightly soluble. Copper(II) sulfide, CuS. Rule 6: Exception. OCopper(II) sulfide, CuS. Rule 3: Most sulfide salts are only slightly soluble. ONo precipitate. c. Potassium phosphate and magnesium nitrate. OMagnesium phosphate, Mg3 (PO4)2. Rule 3: Most phosphate salts are only slightly soluble. Magnesium phosphate, Mg, (PO4)2. Rule 6: Exception. OMagnesium phosphate, Mg, (PO4)2. Rule 6: Most phosphate salts are only slightly soluble. ONo precipitate. d. Sodium carbonate and calcium nitrate. OCalcium carbonate, CaCO3. Rule 3: Most carbonate salts are only slightly soluble. OCalcium carbonate, CaCO3. Rule 6: Exception. OCalcium carbonate, CaCO3. Rule 6: Most carbonate salts are only slightly soluble. ONo precipitate.
a. Magnesium chloride and sodium hydroxide. OMagnesium hydroxide, Mg(OH)2. Rule 5: Most hydroxide salts are only slightly soluble. OMagnesium hydroxide, Mg(OH)2. Rule 5: Exception. OMagnesium hydroxide, Mg(OH)2. Rule 3: Most hydroxide salts are only slightly soluble. ONo precipitate. b. Copper(II) nitrate and ammonium sulfide. Copper(II) sulfide, CuS. Rule 6: Most sulfide salts are only slightly soluble. Copper(II) sulfide, CuS. Rule 6: Exception. OCopper(II) sulfide, CuS. Rule 3: Most sulfide salts are only slightly soluble. ONo precipitate. c. Potassium phosphate and magnesium nitrate. OMagnesium phosphate, Mg3 (PO4)2. Rule 3: Most phosphate salts are only slightly soluble. Magnesium phosphate, Mg, (PO4)2. Rule 6: Exception. OMagnesium phosphate, Mg, (PO4)2. Rule 6: Most phosphate salts are only slightly soluble. ONo precipitate. d. Sodium carbonate and calcium nitrate. OCalcium carbonate, CaCO3. Rule 3: Most carbonate salts are only slightly soluble. OCalcium carbonate, CaCO3. Rule 6: Exception. OCalcium carbonate, CaCO3. Rule 6: Most carbonate salts are only slightly soluble. ONo precipitate.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter17: Solubility And Complex-ion Equilibria
Section: Chapter Questions
Problem 17.15QP: Solubility and Solubility Product You put 0.10-mol samples of KNO3, (NH4)2S, K2S, MnS, AgCl, and...
Related questions
Question
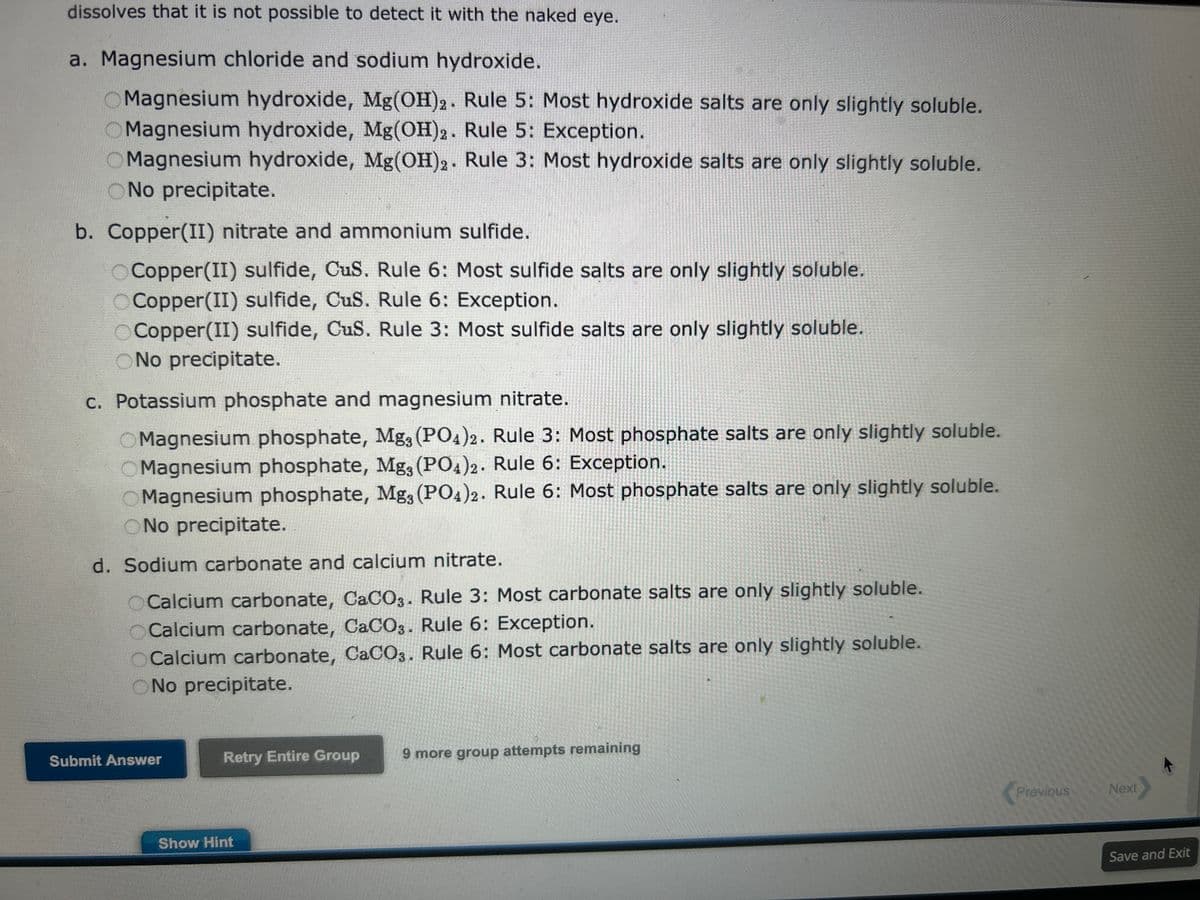
Transcribed Image Text:On the basis of the general solubility rules, predict the identity of the precipitate that forms when aqueous solutions of
the following substances are mixed.
General Rules for Solubility of Ionic Compounds (Salts) in Water at 25
°C.
1. Most nitrate (NO3) salts are soluble.
2. Most salts of
Na+,
K+, and
+
NH4 are soluble.
3. Most chloride salts are soluble. Notable exceptions are
AgCl,
PbCl2, and
Hg₂ Cl₂.
4. Most sulfate salts are soluble. Notable exceptions are
BaSO4,
PbSO4, and
CaSO4.
5. Most hydroxide compounds are only slightly soluble.* The important
exceptions are
NaOH and
KOH.
Ba(OH)2 and
Ca(OH)2 are only moderately soluble.
6. Most sulfide (
S²-), carbonate (
2-
CO3), and phosphate (
3-
PO4³) salts are only slightly soluble. *
*The terms insoluble and slightly soluble really mean the same thing: such a tiny amount
dissolves that it is not possible to detect it with the naked eye.
Show Hint
Previous
Next
Save and
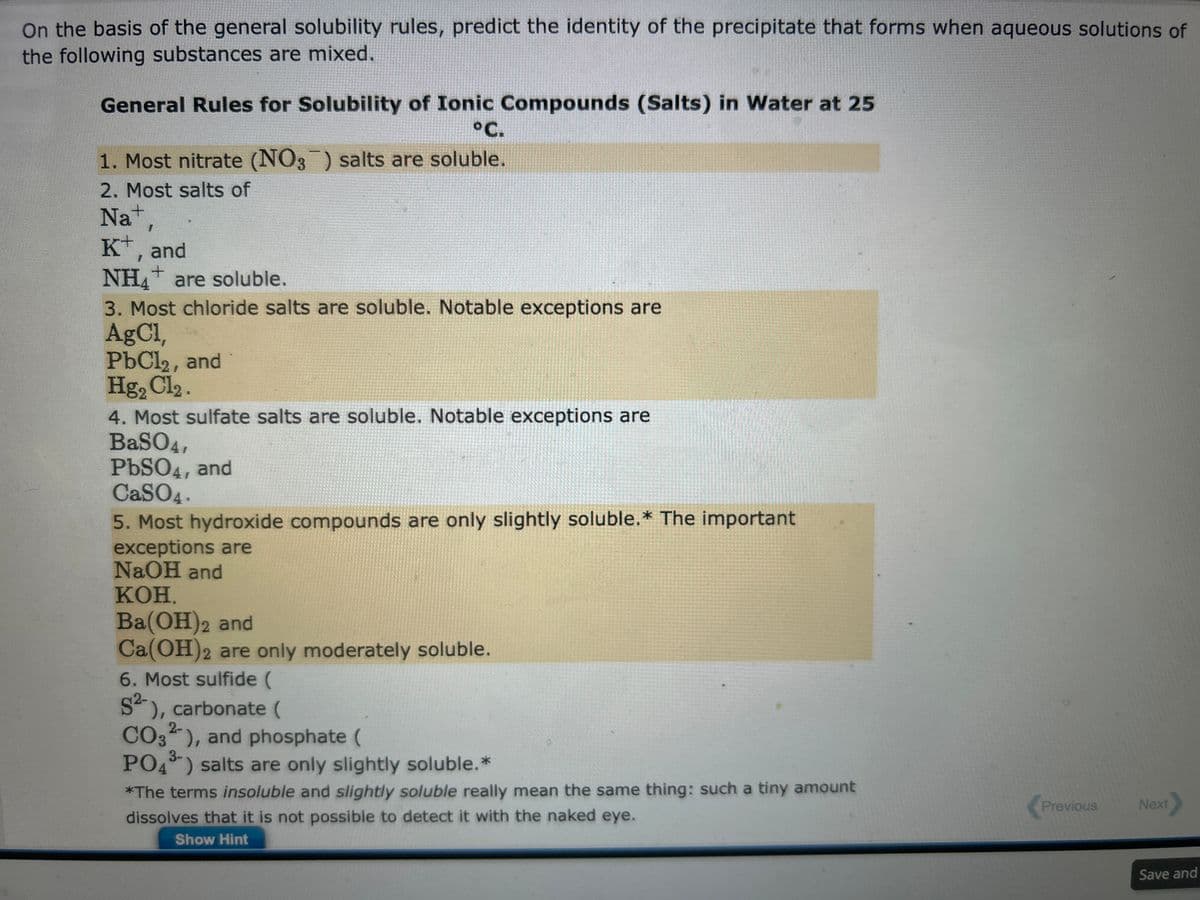
Transcribed Image Text:On the basis of the general solubility rules, predict the identity of the precipitate that forms when aqueous solutions of
the following substances are mixed.
General Rules for Solubility of Ionic Compounds (Salts) in Water at 25
°C.
1. Most nitrate (NO3) salts are soluble.
2. Most salts of
Na+,
K+, and
+
NH4 are soluble.
3. Most chloride salts are soluble. Notable exceptions are
AgCl,
PbCl2, and
Hg₂ Cl₂.
4. Most sulfate salts are soluble. Notable exceptions are
BaSO4,
PbSO4, and
CaSO4.
5. Most hydroxide compounds are only slightly soluble.* The important
exceptions are
NaOH and
KOH.
Ba(OH)2 and
Ca(OH)2 are only moderately soluble.
6. Most sulfide (
S²-), carbonate (
2-
CO3), and phosphate (
3-
PO4³) salts are only slightly soluble. *
*The terms insoluble and slightly soluble really mean the same thing: such a tiny amount
dissolves that it is not possible to detect it with the naked eye.
Show Hint
Previous
Next
Save and
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning