A. TRUE OR FALSE. Write T before each number if the statement is correct statement is incorrect. anu 1. A period is a vertical column of elements in the periodic table. 2. There are 16 groups in a periodic table. 3. John Newlands proposed the periodic table in triads. 4. It is impossible for 2 elements to be located in the same period and group. 5. Antoine Lavoiser arranged element into groups of simple substances that do not decompose by any means. 6. The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. 7. Oxygen is an example of a halogen. 8.Johann Dobereiner made a list of elements arranged by increasing atomic weight in spiral order. 9. Metalloids have shared properties of metal and nonmetals. 10. Malleability is the property of matter being able to be drawn into wires. 11. The only nonmetal that is a liquid at room temperature is bromine. 12. Gold has higher melting point compared to Nitrogen. 13. lonization potential is tthe tendency of an atom to attract a shared pair of electrons towards itself. 14. Lothar Meyer & Dmitri Mendeleev arranged the elements according to its atomic number. 15. Jons Jakob Berzelius proposed a system of chemical symbols based on the first letter of based on their latin name.
A. TRUE OR FALSE. Write T before each number if the statement is correct statement is incorrect. anu 1. A period is a vertical column of elements in the periodic table. 2. There are 16 groups in a periodic table. 3. John Newlands proposed the periodic table in triads. 4. It is impossible for 2 elements to be located in the same period and group. 5. Antoine Lavoiser arranged element into groups of simple substances that do not decompose by any means. 6. The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. 7. Oxygen is an example of a halogen. 8.Johann Dobereiner made a list of elements arranged by increasing atomic weight in spiral order. 9. Metalloids have shared properties of metal and nonmetals. 10. Malleability is the property of matter being able to be drawn into wires. 11. The only nonmetal that is a liquid at room temperature is bromine. 12. Gold has higher melting point compared to Nitrogen. 13. lonization potential is tthe tendency of an atom to attract a shared pair of electrons towards itself. 14. Lothar Meyer & Dmitri Mendeleev arranged the elements according to its atomic number. 15. Jons Jakob Berzelius proposed a system of chemical symbols based on the first letter of based on their latin name.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter1: The Nature Of Chemistry
Section1.13: The Periodic Table
Problem 1.9E
Related questions
Question

Transcribed Image Text:A. TRUE OR FALSE. Write T before each number if the statement is
statement is incorrect.
1. A period is a vertical column of elements in the periodic table.
2. There are 16 groups in a periodic table.
3. John Newlands proposed the periodic table in triads.
4. It is impossible for 2 elements to be located in the same period and group.
5. Antoine Lavoiser arranged element into groups of simple substances that do not
decompose by any means.
_6. The atomic radius of a chemical element is a measure of the size of its atoms, usually the
mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons.
7. Oxygen is an example of a halogen.
8.Johann Dobereiner made a list of elements arranged by increasing atomic weight in
spiral order.
9. Metalloids have shared properties of metal and nonmetals.
10. Malleability is the property of matter being able to be drawn into wires.
11. The only nonmetal that is a liquid at room temperature is bromine.
12. Gold has higher melting point compared to Nitrogen.
13. lonization potential is tthe tendency of an atom to attract a shared pair of electrons
towards itself.
14. Lothar Meyer & Dmitri Mendeleev arranged the elements according to its atomic number.
15. Jons Jakob Berzelius proposed a system of chemical symbols based on the first letter of
me and some based on their latin name.
their
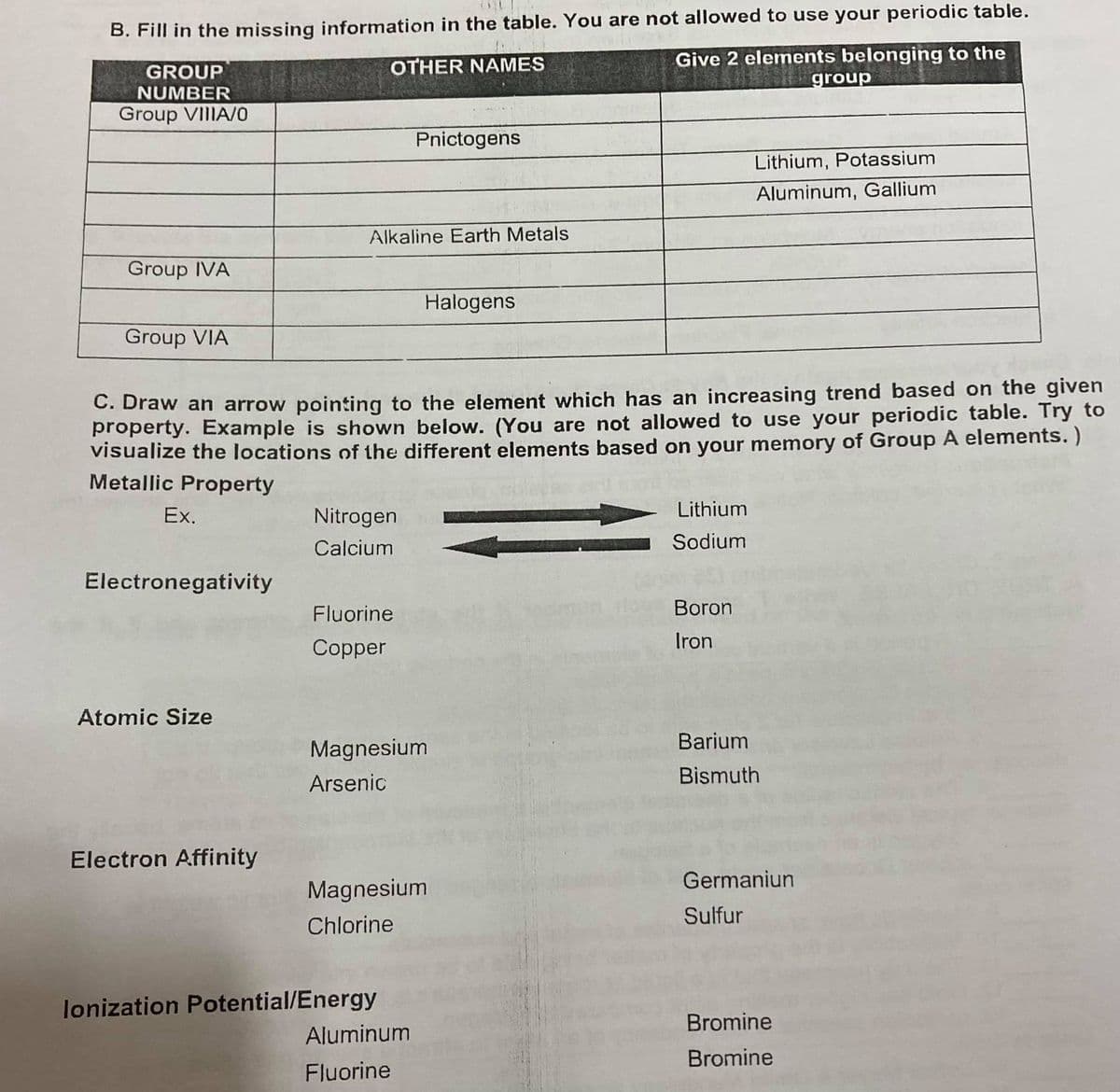
Transcribed Image Text:B. Fill in the missing information in the table. You are not allowed to use your periodic table.
Give 2 elements belonging to the
group
GROUP
OTHER NAMES
NUMBER
Group VIIIA/0
Pnictogens
Lithium, Potassium
Aluminum, Gallium
Alkaline Earth Metals
Group IVA
Halogens
Group VIA
C. Draw an arrow pointing to the element which has an increasing trend based on the given
property. Example is shown below. (You are not allowed to use your periodic table. Try to
visualize the locations of the different elements based on your memory of Group A elements. )
Metallic Property
Ex.
Nitrogen
Lithium
Calcium
Sodium
Electronegativity
Fluorine
Boron
Iron
Copper
Atomic Size
Barium
Magnesium
Arsenic
Bismuth
Electron Affinity
Germaniun
Magnesium
Sulfur
Chlorine
lonization Potential/Energy
Aluminum
Bromine
Bromine
Fluorine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co