a. What is the hybridization at each of the labeled carbon atoms? b. What is the electron-pair geometry at each of the labeled carbon atoms? c. Which orbitals are responsible for the following bonds? Indicate which types of orbitals are used (i.e. s, p, sp?, etc.) and which types of bonds are formed or T). i. Bond a-b ii. Bond b-c iii. Bond d-e iv. Bond between carbon b and a hydrogen bound to it.
a. What is the hybridization at each of the labeled carbon atoms? b. What is the electron-pair geometry at each of the labeled carbon atoms? c. Which orbitals are responsible for the following bonds? Indicate which types of orbitals are used (i.e. s, p, sp?, etc.) and which types of bonds are formed or T). i. Bond a-b ii. Bond b-c iii. Bond d-e iv. Bond between carbon b and a hydrogen bound to it.
Chapter9: Covalent Bonding: Orbitals
Section: Chapter Questions
Problem 41E: Consider the following molecular orbitals formed from the combination of two hydrogen 1s orbitals:...
Related questions
Question
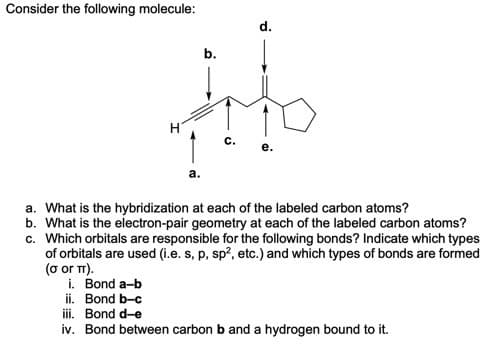
Transcribed Image Text:Consider the following molecule:
d.
b.
c.
a. What is the hybridization at each of the labeled carbon atoms?
b. What is the electron-pair geometry at each of the labeled carbon atoms?
c. Which orbitals are responsible for the following bonds? Indicate which types
of orbitals are used (i.e. s, p, sp?, etc.) and which types of bonds are formed
(o or T).
i. Bond a-b
ii. Bond b-c
iii. Bond d-e
iv. Bond between carbon b and a hydrogen bound to it.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning