a. Which type of chromatography based on the polarity of the stationary phase was employed in the experiment? b. Identify the limiting and excess reactants. c. Which product appeared after 30 minutes?
a. Which type of chromatography based on the polarity of the stationary phase was employed in the experiment? b. Identify the limiting and excess reactants. c. Which product appeared after 30 minutes?
Chapter33: High-performance Liquid Chromatography
Section: Chapter Questions
Problem 33.12QAP
Related questions
Question
Paper chromatography
(show complete solution)
Transcribed Image Text:Paper Chromatography
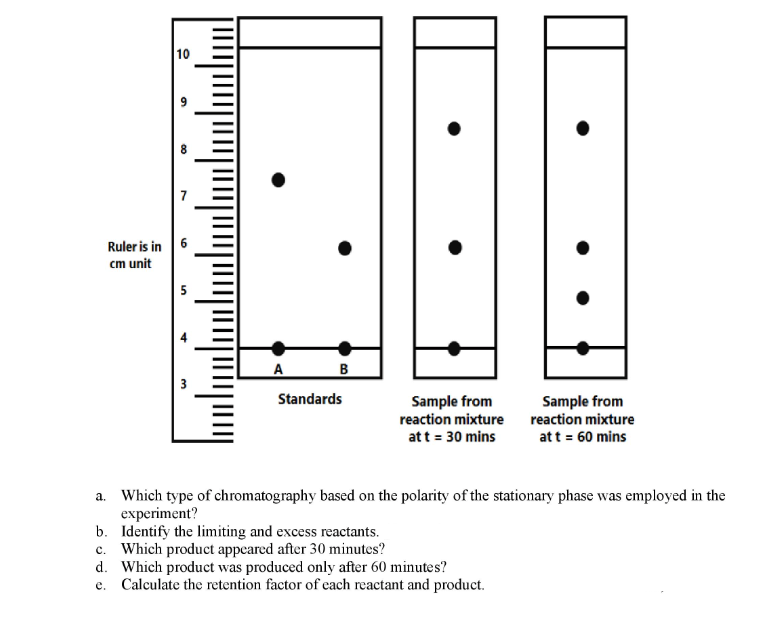
monitored using paper chromatography. Compounds A and B were mixed and a sample of the solution
was spotted every thirty minutes. The same stationary phase and mobile phase were used throughout the
experiment. The figure below shows chromatograms of standards A and B, and the sample at different time
points. Hint: C is less polar than D.
The progress of a hypothetical reaction: A + B → C + D, was
Transcribed Image Text:10
7
Ruler is in 6
cm unit
5
A
B
3
Standards
Sample from
reaction mixture
att = 30 mins
Sample from
reaction mixture
att = 60 mins
a. Which type of chromatography based on the polarity of the stationary phase was employed in the
experiment?
b. Identify the limiting and excess reactants.
c. Which product appeared after 30 minutes?
d. Which product was produced only after 60 minutes?
e. Calculate the retention factor of each reactant and product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
