a.edu/d2l/Ims/quizzing/user/attempt/quiz_start_frame_auto.d2l?O%3D220518&isprv3&drc%3D1&qi=20426&cfql%3D0&dnb3D0 orcement nna Claiborne: Attempt 2 Write the Lewis structures of the indicated chemical substance and use it to answer the other questions. E. CO a) Draw the Lewis structure. b) Indicate which atoms in the species need what hybrid orbitals in order to explain the bonding in the molecule or ion. (Please write for example: sp4 hybrid orbitals on Na.) Use proper spelling or symbol for all elements. AV C) 1st type of bond: "There is a bond between the _-_ atom and the atom formed by the overlap of a orbital on the atom.) (Fill in the blanks from left to right). atom and the orbital on the triple bond C. a :
a.edu/d2l/Ims/quizzing/user/attempt/quiz_start_frame_auto.d2l?O%3D220518&isprv3&drc%3D1&qi=20426&cfql%3D0&dnb3D0 orcement nna Claiborne: Attempt 2 Write the Lewis structures of the indicated chemical substance and use it to answer the other questions. E. CO a) Draw the Lewis structure. b) Indicate which atoms in the species need what hybrid orbitals in order to explain the bonding in the molecule or ion. (Please write for example: sp4 hybrid orbitals on Na.) Use proper spelling or symbol for all elements. AV C) 1st type of bond: "There is a bond between the _-_ atom and the atom formed by the overlap of a orbital on the atom.) (Fill in the blanks from left to right). atom and the orbital on the triple bond C. a :
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter2: Lewis Structures
Section: Chapter Questions
Problem 20CTQ
Related questions
Question
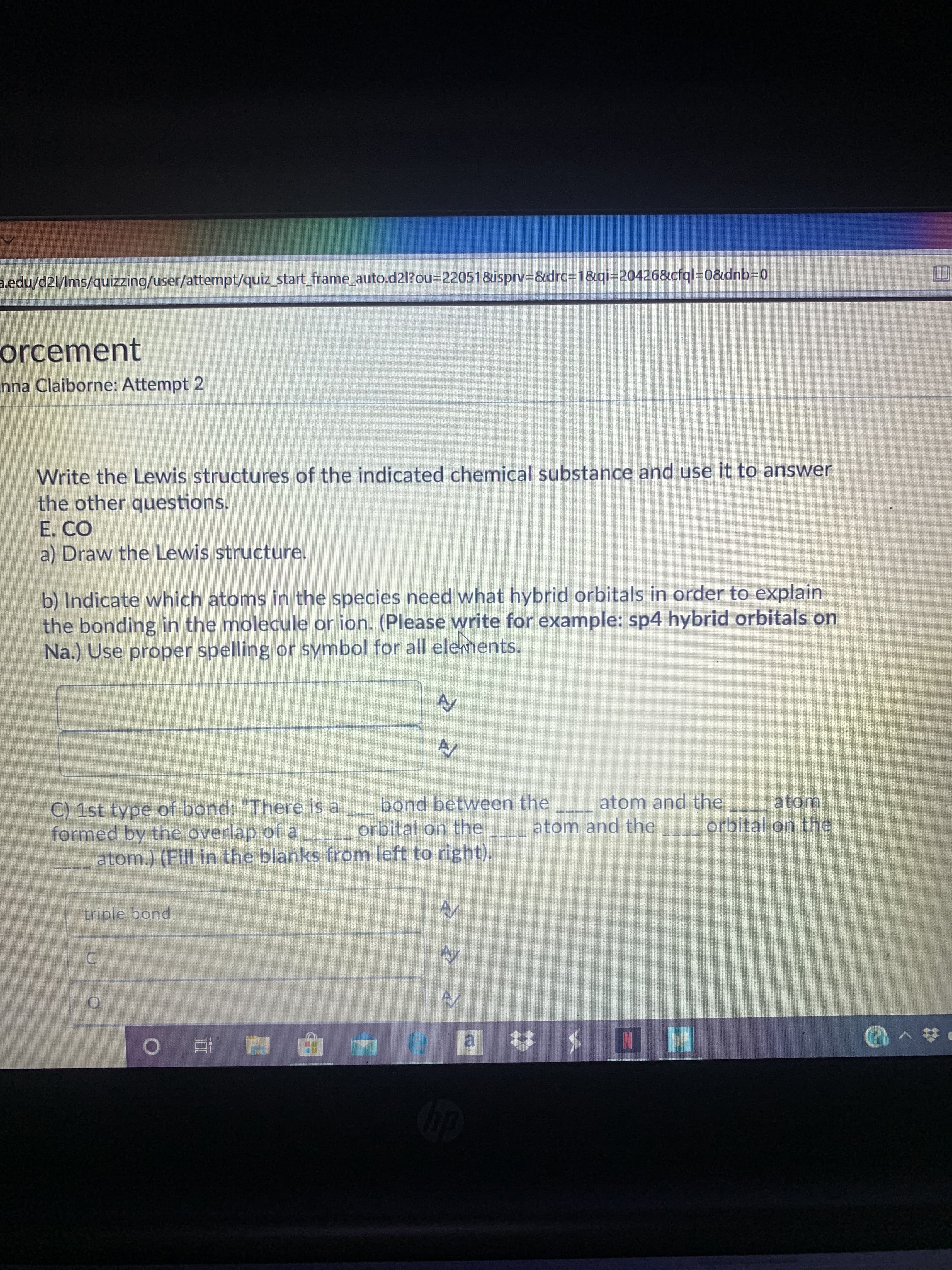
Transcribed Image Text:a.edu/d2l/Ims/quizzing/user/attempt/quiz_start_frame_auto.d2l?O%3D220518&isprv3&drc%3D1&qi=20426&cfql%3D0&dnb3D0
orcement
nna Claiborne: Attempt 2
Write the Lewis structures of the indicated chemical substance and use it to answer
the other questions.
E. CO
a) Draw the Lewis structure.
b) Indicate which atoms in the species need what hybrid orbitals in order to explain
the bonding in the molecule or ion. (Please write for example: sp4 hybrid orbitals on
Na.) Use proper spelling or symbol for all elements.
AV
C) 1st type of bond: "There is a bond between the _-_ atom and the atom
formed by the overlap of a orbital on the
atom.) (Fill in the blanks from left to right).
atom and the
orbital on the
triple bond
C.
a :
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning