According to the bohr model, an atom with 7 electrons has how many PAIRS of electrons? Type your answer. An isotope is an element of the same type but having differing numbers of. (choose all that apply) identities electrons mass neutrons protons According to the bohr model of an atom with 5 electrons, how many electrons are in the second energy level (ring)? Type your answer. Element "X" has a mass# of 64. How many neutrons does it have if its atomic# is 29 Type your answer.
According to the bohr model, an atom with 7 electrons has how many PAIRS of electrons? Type your answer. An isotope is an element of the same type but having differing numbers of. (choose all that apply) identities electrons mass neutrons protons According to the bohr model of an atom with 5 electrons, how many electrons are in the second energy level (ring)? Type your answer. Element "X" has a mass# of 64. How many neutrons does it have if its atomic# is 29 Type your answer.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter11: Atomic Theory :the Quantum Model Of The Atom
Section: Chapter Questions
Problem 8E: In the Bohr model of the hydrogen atom, the electron occupies distinct energy states. In one...
Related questions
Question
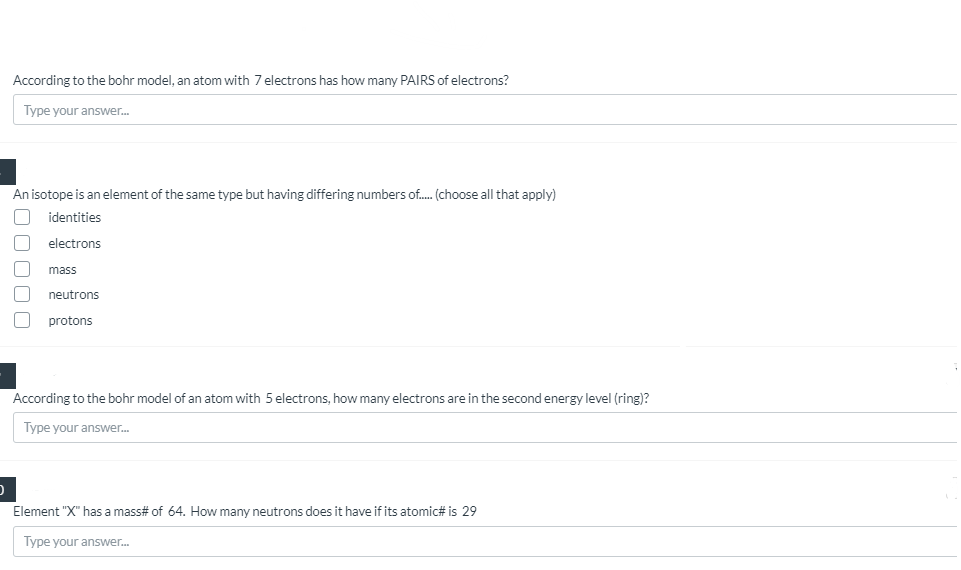
Transcribed Image Text:According to the bohr model, an atom with 7 electrons has how many PAIRS of electrons?
Type your answer.
An isotope is an element of the same type but having differing numbers of. (choose all that apply)
identities
electrons
mass
neutrons
protons
According to the bohr model of an atom with 5 electrons, how many electrons are in the second energy level (ring)?
Type your answer.
Element "X" has a mass# of 64. How many neutrons does it have if its atomic# is 29
Type your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning