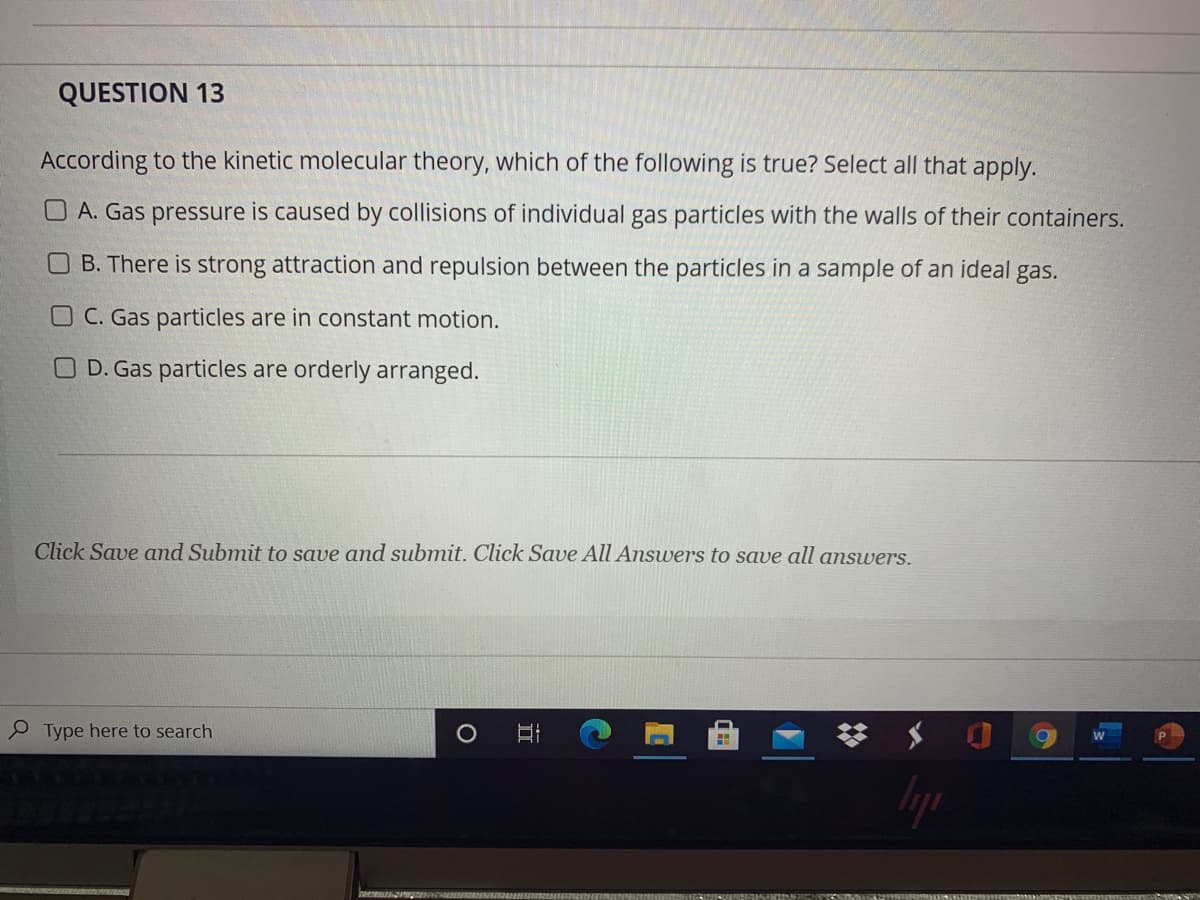
According to the kinetic molecular theory, which of the following is true? Select all that apply. O A. Gas pressure is caused by collisions of individual gas particles with the walls of their containers. O B. There is strong attraction and repulsion between the particles in a sample of an ideal gas. O C. Gas particles are in constant motion. O D. Gas particles are orderly arranged.
According to the kinetic molecular theory, which of the following is true? Select all that apply. O A. Gas pressure is caused by collisions of individual gas particles with the walls of their containers. O B. There is strong attraction and repulsion between the particles in a sample of an ideal gas. O C. Gas particles are in constant motion. O D. Gas particles are orderly arranged.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 7STP
Related questions
Question
Transcribed Image Text:QUESTION 13
According to the kinetic molecular theory, which of the following is true? Select all that apply.
O A. Gas pressure is caused by collisions of individual gas particles with the walls of their containers.
B. There is strong attraction and repulsion between the particles in a sample of an ideal gas.
O C. Gas particles are in constant motion.
O D. Gas particles are orderly arranged.
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
P Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning